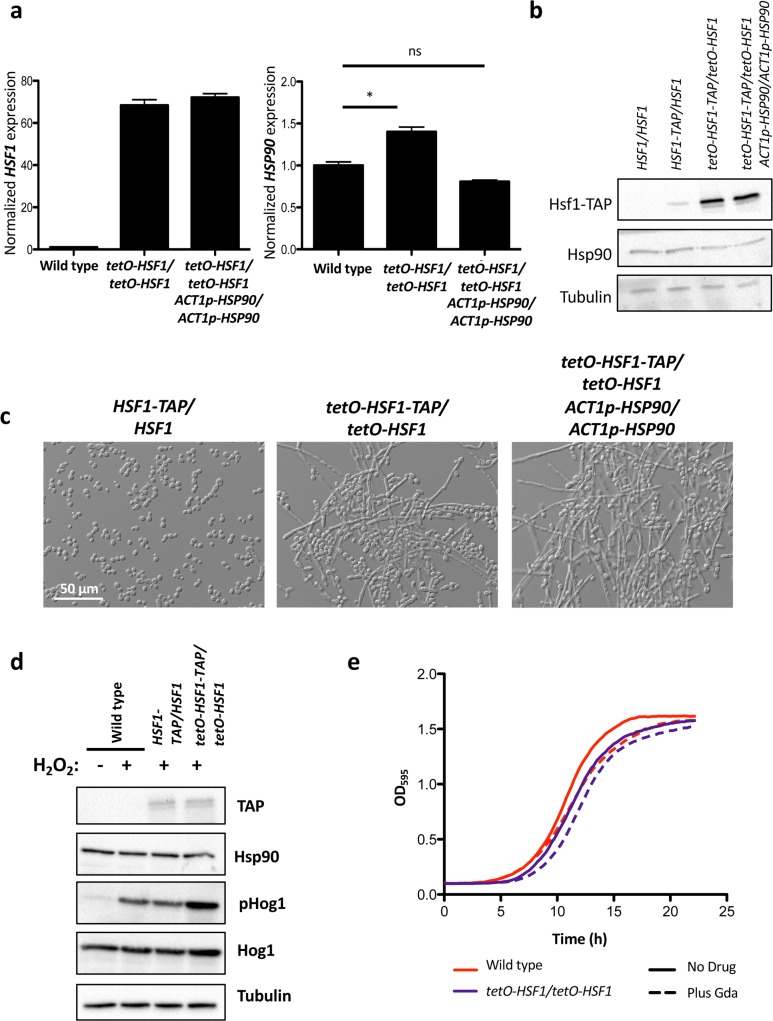
Fig 6. HSF1 overexpression induces filamentation independently of changes in HSP90 transcript or protein levels, and function.
a) To monitor the effects of HSF1 overexpression on filamentation independently of changes in HSP90 expression, we engineered strains where HSP90 is under the control of a constitutive promoter, ACT1p, in the tetO-HSF1/tetO-HSF1 strain. HSF1 and HSP90 transcript levels were normalized to ACT1 and GPD1. Data are means +/- standard error of the means for triplicate samples. In the ACT1p-HSP90 strain, HSF1 levels are overexpressed (left panel) but HSP90 levels do not differ substantially from the wild-type levels (right panel). * indicates P value <0.05, ns indicates no significant difference, unpaired t test. b) Western blot analysis shows that overexpression of HSF1 does not impact the levels of Hsp90 protein. Tubulin serves as loading control. c) HSF1 overexpression induces filamentation independently of changes in HSP90 expression. Strains were grown in the absence of DOX at 30°C. d) Western blot analysis was performed to assay if HSF1 overexpression compromises Hsp90 function by monitoring the Hsp90 client protein Hog1. Cells were treated with 5 mM hydrogen peroxide (H2O2) for 10 minutes to induce oxidative stress before protein extraction. Overexpression of HSF1 does not affect the stability of Hog1, nor does it block its activation. Tubulin serves as a loading control. e) HSF1 overexpression does not cause hypersensitivity to the Hsp90 inhibitor geldanamycin (Gda). Growth curves were generated by measuring the optical density of cells grown in the absence and presence of 20 μg/mL DOX in the presence of 3.13 μM geldanamycin. Optical density measurements at 595 nm were taken every 15 minutes with a TECAN plate reader. Experiment was performed in biological quadruplicate, with one representative graph shown.