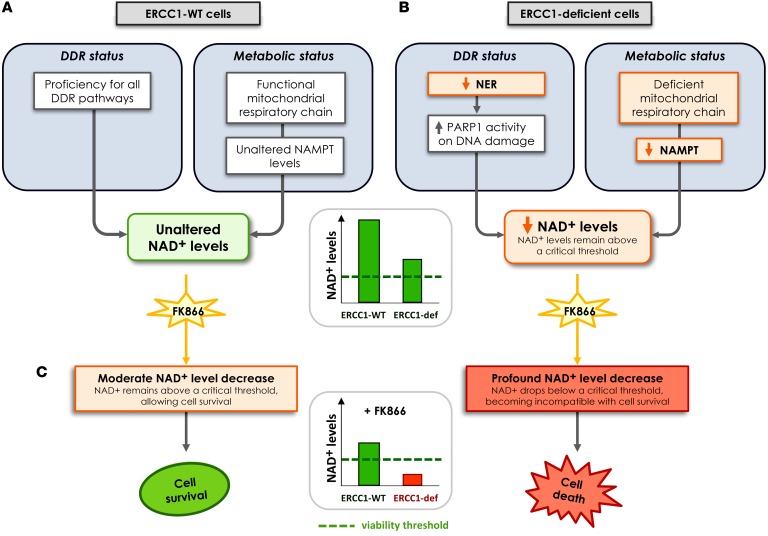
Figure 10. Proposed model for the synthetic lethality between NAMPT inhibition and ERCC1 deficiency in NSCLC cells.
(A) ERCC1-proficient cells have a proficient DNA damage repair status and no metabolic alteration in the NAD+ biosynthesis pathway. (B) Loss of ERCC1 induces decreased NER activity and dependency upon PARP1 activation for the repair of DNA lesions, thereby causing NAD+ consumption. Chronic ERCC1 deficiency/PARP1 activation is associated with metabolic defects including decreased NAD+ and NAMPT levels, as well as alterations in the mitochondrial respiratory chain. In this scenario, NAD+ levels are low, but remain above a threshold that allows ERCC1-deficient cells to survive and proliferate. (C) Treatment with NAMPT inhibitors blocks the NAD+ recycling pathway and causes an acute drop in NAD+ levels. In ERCC1-proficient cells, NAD+ levels remain above a critical threshold compatible with cell survival. In ERCC1-deficient cells, this acute drop in NAD+ levels outstrips the cellular NAD+ replenishment capacities and decompensates a fragile equilibrium; NAD+ levels drop below a threshold compatible with cell survival, which triggers cell death. In this model, NAMPT and ERCC1 are synthetic lethal, as the combination of ERCC1 deficiency with NAMPT inhibition causes cell death, whereas each perturbation in isolation (i.e., ERCC1 deficiency or NAMPT inhibition) does not. Arrows represent a relationship and not a demonstrated causality link. DDR, DNA damage response; ERCC1-def, ERCC1-deficient; NER, nucleotide excision repair.