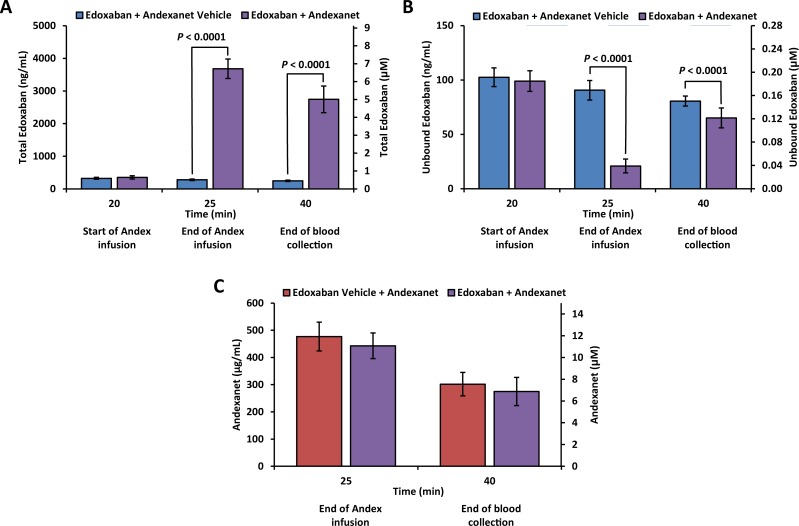
Fig 7. Change in pharmacokinetic parameters following administration of andexanet in edoxaban-anticoagulated rabbits.
(A) Total edoxaban plasma concentration. (B) Unbound edoxaban plasma concentration. Total and unbound (pharmacologically active) edoxaban plasma levels were determined by LC/MS/MS with a turbo-ion spray source using an internal standard (edoxaban-D6) as described in Materials and Methods. Pharmacologically active unbound edoxaban was separated using an HTD96b apparatus at 37°C for approximately 4 hours with gentle vortexing. (C) Andexanet plasma concentration was determined by ELISA with paired antibodies recognizing human FX/FXa. Andexanet standard was freshly prepared in blocking buffer with the same lot material used in this study for 8-point curve (0–200 ng/mL). Data are plotted as mean ± standard deviation.