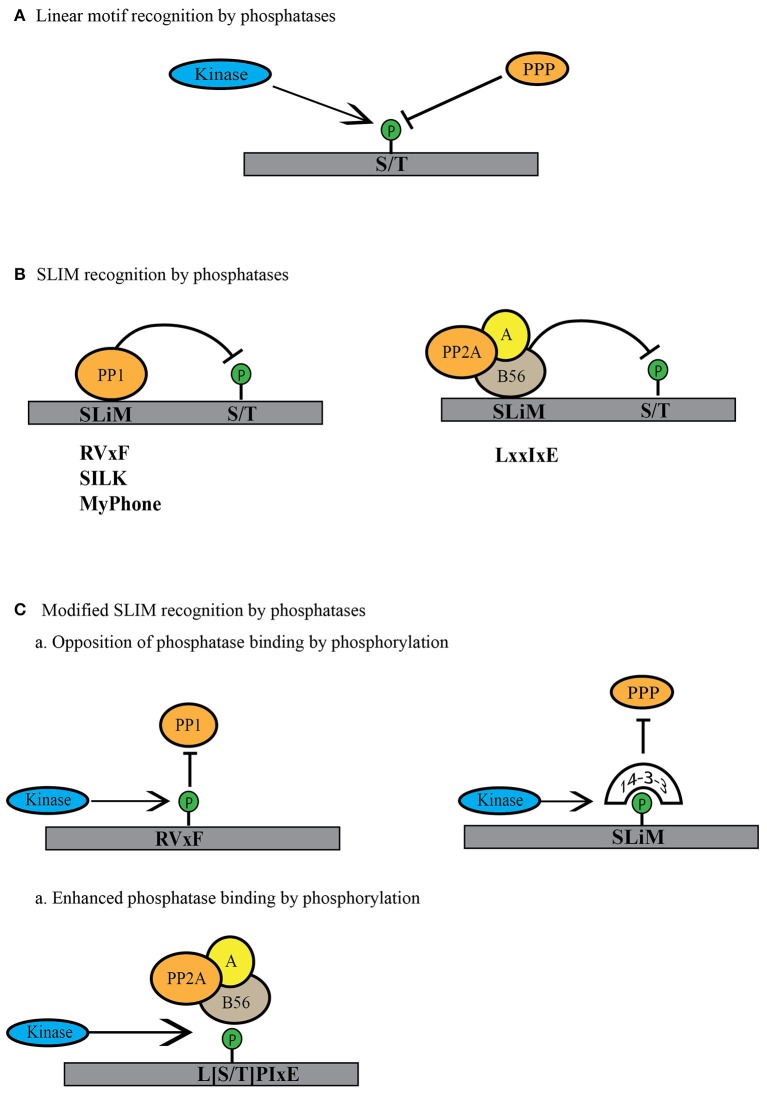
Figure 3.
Modes of linear motif recognition by protein phosphatases. (A) Linear motifs can dictate the binding preferences of PPP family phosphatases. This can include a preference for the phosphosite (serine or threonine) or a preference for the residues surrounding the phosphosite (basic, acidic or proline-directed). (B) PPPs recognize short linear motifs (SLiMs) in regulatory proteins or substrates. PPP binding via the SLiMs helps them recognize and bind the regulatory proteins or the substrates to dephosphorylate them. PP1 is known to bind through RVxF, SILK and MyPhone motifs to its regulatory proteins (shown in gray). B56 regulatory subunit of PP2A binds through LxxIxE motifs to its substrates (shown in gray). (C) Phosphatase activity can be regulated by modulation of the SLiM motif. Phosphorylation within or near the SliM sequence can lead to decreased phosphatase binding either by direct blocking in the case of PP1 or indirectly by binding to phospho-binding 14-3-3 proteins to block the site of interaction. In case of PP2A-B56 SLiM, this phosphorylation can enhance phosphatase activity toward substrate by increasing the affinity for phosphorylated SLiM.