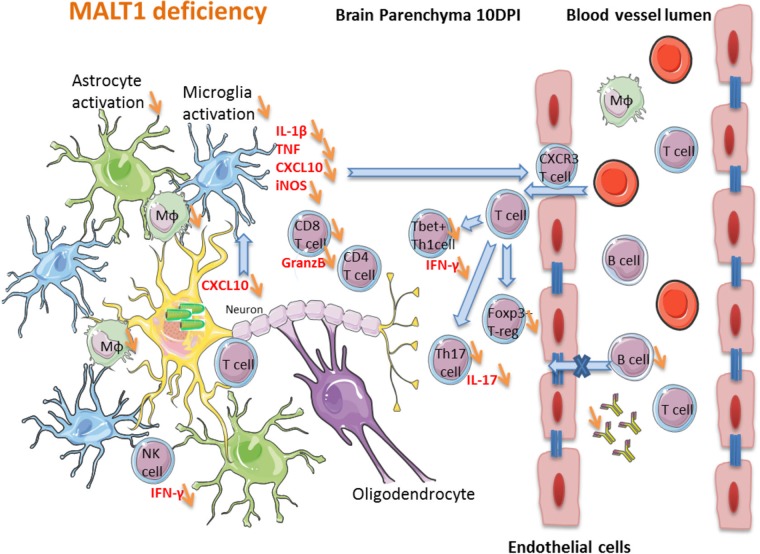
FIG 11.
Hypothetical model of the impact of MALT1 inactivation on ERA virus-induced immune responses in the brain. In MALT1+/+ mice, virus-infected neuronal cells produce CXCL10 (as shown in other studies), leading to the activation of microglia and recruitment of CXCR3-expressing T cells and NK cells to the brain parenchyma. Activated microglia and astrocytes start to produce proinflammatory cytokines, iNOS and CXCL10, which amplifies the recruitment and activation of several immune cell types. Activated macrophages migrate to the cervical lymph nodes, where they serve as antigen-presenting cells for naive T cells and trigger the differentiation to effector T cells, mainly CD4+ Th1 and Th17 subsets and cytotoxic CD8+ T cells. Only activated T cells can infiltrate the brain. IFN-γ-producing CD8+ T cells are the main T cells recruited to the site of infection. Th1 cells and CD8+ T cells can further activate macrophages by IFN-γ production, and antigen-presenting cells can reactivate the T cells that have entered the brain. CD8+ T cells also produce granzyme B, mediating their cytotoxic activity and virus clearance. Th1 cells provide help for B cell activation and immunoglobulin production outside the brain, whereas Th17 cells produce IL-17, contributing to the activation of microglia and astrocytes, as well as enhancing blood-brain barrier permeability. The impact of MALT1 deficiency is represented by orange arrows. MALT1 deficiency in T cells reduces their activation and differentiation by antigen-presenting cells in the periphery, resulting in less effector T cells entering the brain. This not only results in less IFN-γ and IL-17 production but also lowers T cell help to activate B cells and CD8+ cytotoxic T cells, leading to defective production of virus neutralizing antibodies and reduced killing of virus-infected cells. As a consequence, viral load in the brain increases, exacerbating disease development.