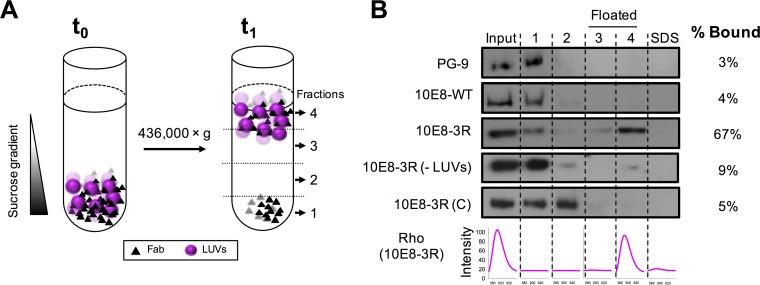
FIG 2.
Partitioning of Fabs into membranes measured by a vesicle flotation assay. (A) Diagram illustrating the method. Fabs (1.5 μM) were incubated with Rho-PE-labeled VL LUVs (1.5 mM) and subsequently deposited onto a stepwise sucrose gradient (time zero [t0]). After ultracentrifugation (t1), four different fractions were collected based on their densities. (B) Partitioning of Fab 10E8 and its derivatives into VL LUVs. The presence of vesicles and Fab in the different fractions was probed by rhodamine emission and Western blotting, respectively. VL LUVs (emission spectra at the bottom right) were found in the third and fourth fractions (i.e., floated fractions). An additional fraction, employing SDS, was collected to recover the material attached to the surface of the tube. Analyzed samples include (from top to bottom) PG9, an anti-gp120 antibody used as a negative control; 10E8-WT; 10E8-3R; 10E8-3R(−LUVs), a 10E8-R3 sample centrifuged in the absence of VL vesicles; and 10E8-3R(C), a control Fab containing a 3R mutation within the constant domain. The values displayed on the right correspond to the percentages of antibody found cofloating with vesicles, calculated by densitometry.