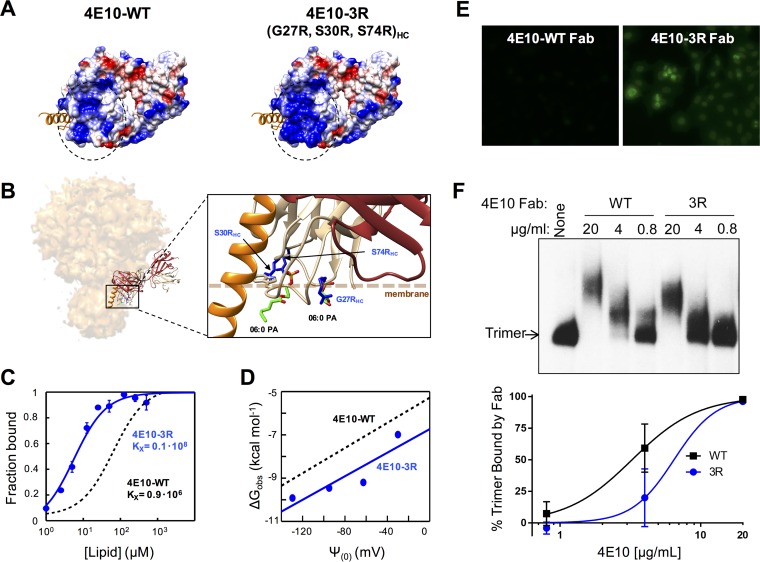
FIG 9.
Design, membrane binding, and polyreactivity of the 4E10-3R antibody. (A) Surface density charge representation (bottom views) of Fab 4E10-WT (PDB accession number 2FX7) and mutant Fab 4E10-3R (rendered by the introduction of the G27R/S30R/S74R triple substitution into the heavy chain). (B) Model for interaction with an Env trimer and detailed view of the mutated positions (in blue) relative to the membrane (dotted line). Bound dihexanoyl phosphatidic acid molecules (06:0 PA) (colored by atoms) and the MPER helix (in orange) were obtained from structures under PDB accession numbers 4XBG and 5GHW, respectively. The light and heavy chains of the Fabs are shown in brown and tan, respectively. (C) Binding of 4E10-3R (blue traces and symbols) to DOPC-DOPS (50:50) LUVs monitored by changes in NBD fluorescence. The black dotted line follows the binding of 4E10-WT. The fraction of Fab bound as a function of the concentration of lipid accessible (half the total lipid concentration) was plotted after titration of NBD-labeled Fab with increasing concentrations of liposomes. Conditions are otherwise as described in the legend of Fig. 4. (D) Plots of the free energy of partitioning versus the membrane surface potential in the above-described lipid vesicles. The black dotted line adjusts to 4E10-WT data and is included for comparison. (E) Immunofluorescence staining experiment 2 using 4E10-3R against HEp-2 cells. Fab 4E10-WT and mutant Fab 4E10-3R were used at a concentration of 25 μg/ml. Images shown are at a ×200 magnification. (F) Binding of Fab 4E10-3R to native Env trimers on virions. (Top) HIV-1 virions displaying Comb-mut Env were incubated with Fabs 4E10-WT or 4E10-3R, and Env-Fab complexes were resolved by using BN-PAGE Western blotting. (Bottom) The percentage of the trimer bound by antibody was calculated by comparing the intensities of the unliganded trimer band in the presence and absence of Fab. Conditions are otherwise as described in the legend of Fig. 8.