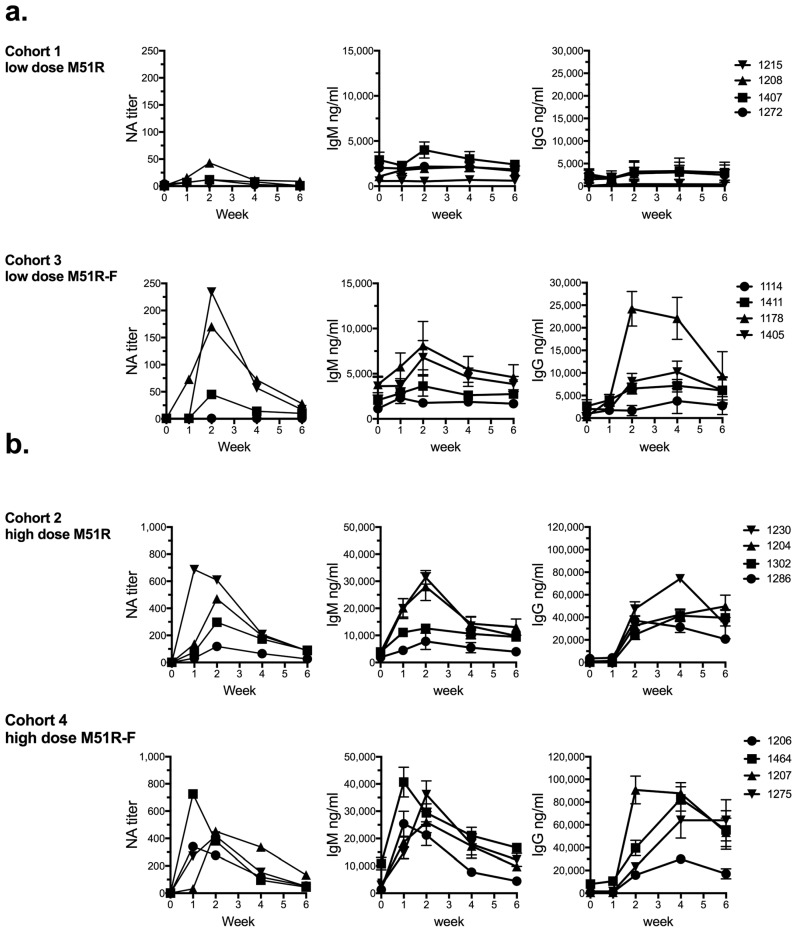
Figure 1.
Humoral immune response. Plasmas were analyzed before vaccination (week 0, pre-immune) and at weeks 1, 2, 4 and 6 post-vaccination for anti-VSV antibodies. Neutralizing antibody (NA) titer (left panel) was defined as the dilution of serum that inhibited infection of mouse EL4 cells with M51R-eGFP by 50%. IgM (middle panel) and IgG (right panel) levels are expressed in ng/mL (mean ± SD, triplicate values), measured by ELISA with extrapolation from standard curves. Data for low dose (a) and high dose (b) vaccinations with M51R and M51R-F are shown. Note scale differences for low and high dose data. Statistical significance was determined by two-factor analysis of variance with cohort and time as the two factors. For all three antibody types, statistical significance of p < 0.05 (after correction for multiple comparisons) was obtained for comparisons of cohort 1 versus cohort 3 (low dose M51R vs. M51R-F), cohort 1 versus cohort 2 (low dose M51R vs. high dose M51R), and cohort 3 versus cohort 4 (low dose M51R-F vs. high dose M51R-F), but not for cohort 2 versus cohort 4 (high dose M51R vs. high dose M51R-F). The data shown represent 1 of 2 analyses with similar results performed on plasma from each animal.