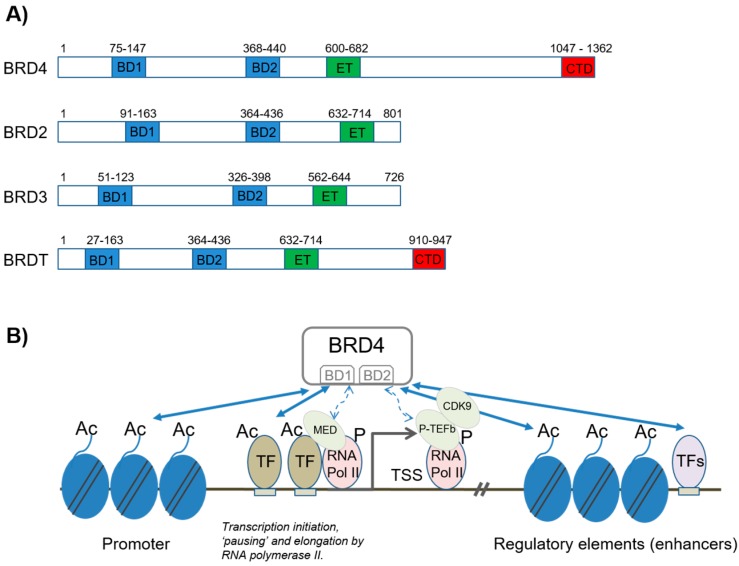
Figure 1.
Schematic of domain organization of BET (bromodomain and extra-terminal domain) family proteins and the function of BRD4 in the regulation of promoter and enhancer activity. (A) Domain organization of human BET family members BRD4, BRD2, BRD3, and BRDT, as indicated. BET proteins contain two bromodomains (BD1 and BD2, respectively) and an extra-terminal domain (ET). BRD4 (long form) contains a carboxyterminal domain (CTD) that is not present in the other BET family members; (B) Schematic representation of the functions of BET family member BRD4 in the regulation of promoter and enhancer function (includes “super-enhancers”; see text for details). Through its BD1 and BD2 domains, BRD4 binds to acetylated lysines (Ac) in histones or transcription factors (TF). The binding of acetylated histones by BRD4, at transcription start sites (TSS), mediates transcriptional co-activation and elongation via RNA polymerase II (RNA pol II) and Mediator (Med) and pTEFb signaling complexes, respectively (see text for details). BRD4 can also bind acetylated lysines in histones or TF in enhancer elements, thereby contributing to long-range control of gene activity (see text for details). TF binding sites are depicted as horizontal rectangles.