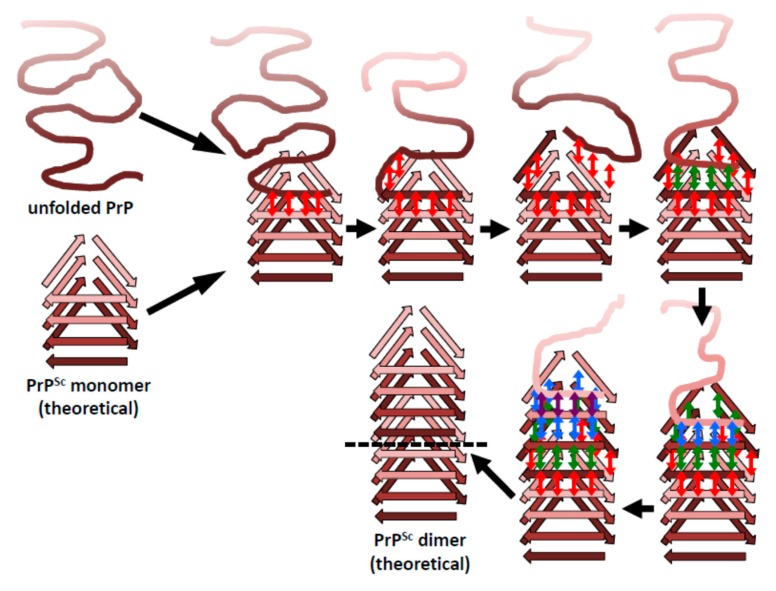
Figure 4.
The β-solenoid architecture of PrPSc suggests a mechanism to template incoming molecules of unfolded PrP onto the existing β-solenoid fold to generate a copy of itself. For simplicity, this mechanistic model is based on a head-to-tail arrangement. An incoming molecule of unfolded PrP would interact with an uncapped β-solenoid surface and adopt a β-strand conformation by forming backbone hydrogen-bonds (red arrows). Once the first rung of a nascent β-solenoid configuration has been formed, it would self-template successive rungs of β-solenoid structure using the same mechanism (green, blue, and purple arrows, respectively). Once the fourth and final rung has been templated, a new molecule of PrPSc is formed and the original template surface has been re-created. Any mutations facilitating unfolding of PrPC would lead to increased chances of propagation events.