Table 1. Screening of potential bidentate chiral auxiliaries and optimization of the reaction conditions a .
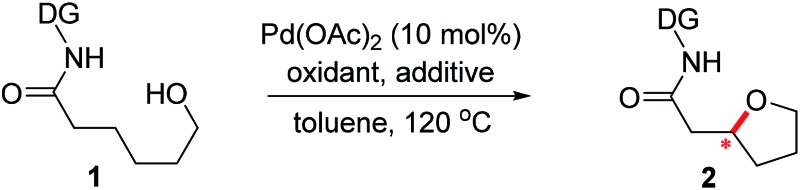
| ||||
| Entry | DG | Oxidant (equiv.) | Additive (equiv.) | Yield c (d.r.) d |
| 1 | 3a | PhI(OAc)2 (2) | AcOH (4) | 65% (4.5 : 1) |
| 2 | 3b | PhI(OAc)2 (2) | AcOH (4) | 64% (3.3 : 1) |
| 3 | 3c | PhI(OAc)2 (2) | AcOH (4) | 64% (6.2 : 1) |
| 4 | 3d | PhI(OAc)2 (2) | AcOH (4) | 63% (26 : 1) |
| 5 | 3e | PhI(OAc)2 (2) | AcOH (4) | 43% (19 : 1) |
| 6 | 3d | K2S2O8 (2) | AcOH (4) | NR |
| 7 | 3d | DMP (2) | AcOH (4) | NR |
| 8 | 3d | PhI(OAc)2 (2) | — | 46% (8.3 : 1) |
| 9 | 3d | PhI(OAc)2 (2) | AgOAc (2) | 39% (6.7 : 1) |
| 10 | 3d | PhI(OAc)2 (2) | PivOH (4) | 58% (23 : 1) |
| 11 | 3d | PhI(OAc)2 (3) | AcOH (4) | 66% (26 : 1) |
| 12 b | 3d | PhI(OAc)2 (3) | AcOH (4) | 71% (30 : 1) |
aSubstrate (1.0 equiv.), Pd(OAc)2 (10 mol%), oxidant, and additive in toluene (0.1 M) at 120 °C for 10 h.
bThe reaction was carried out in a co-solvent system (toluene : EtOH = 10 : 1).
cThe isolated yields of products.
dThe d.r. was determined by HPLC analysis. DMP = Dess–Martin periodinane. NR = no reaction.
