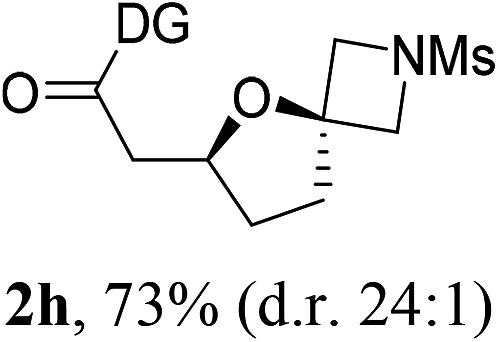
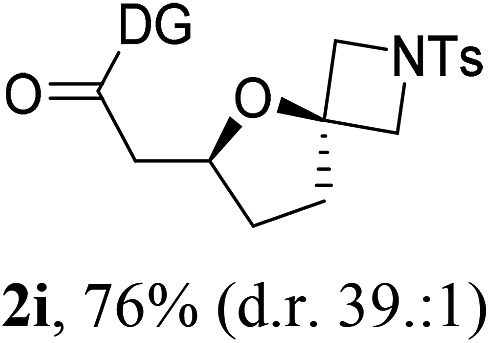
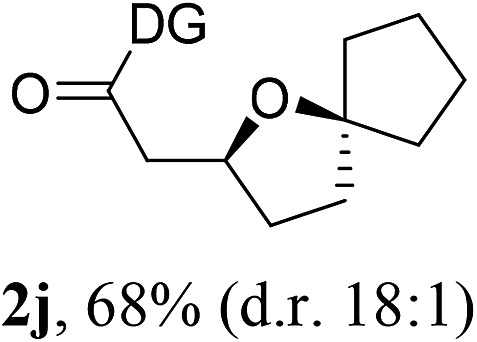
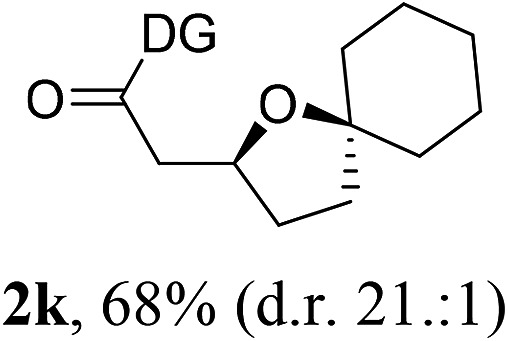
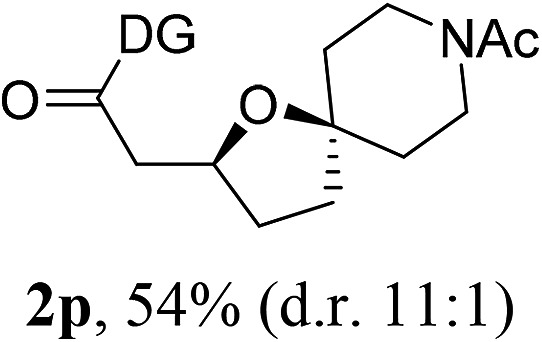
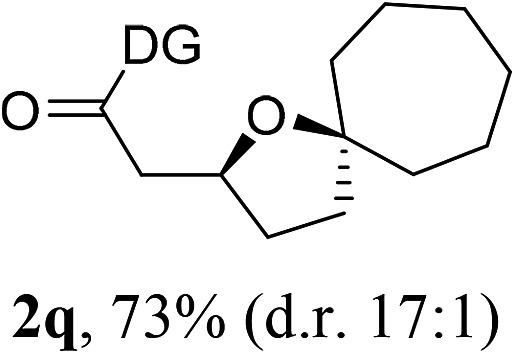
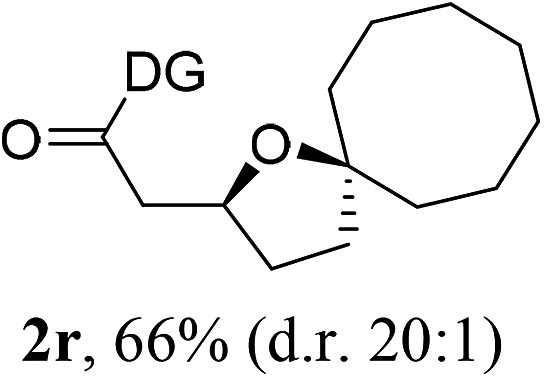
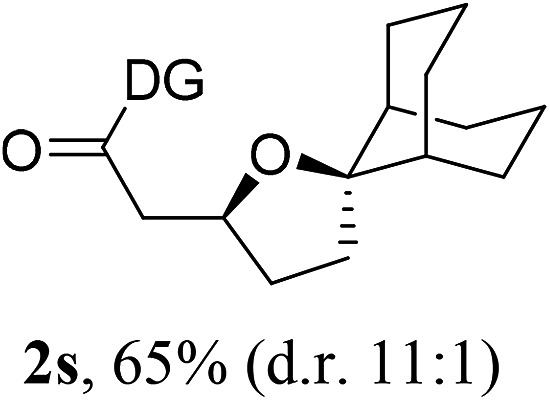
Table 2. Substrate scope a .
| ||
|
|
|
|
|
|
|
|
|
|
|
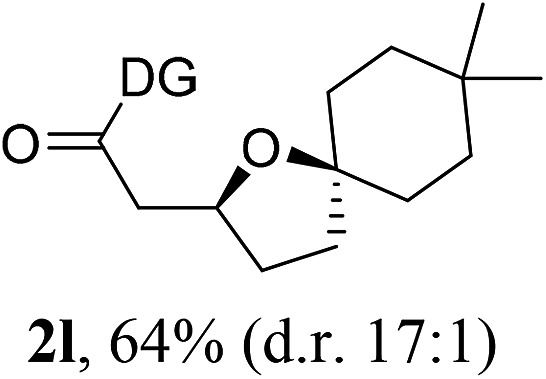
|
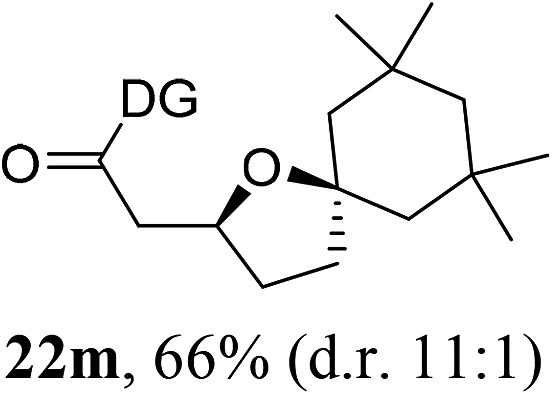
|
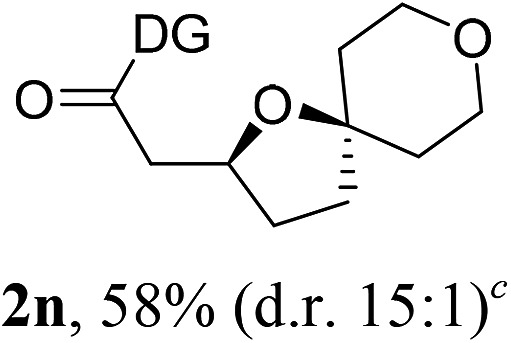
|
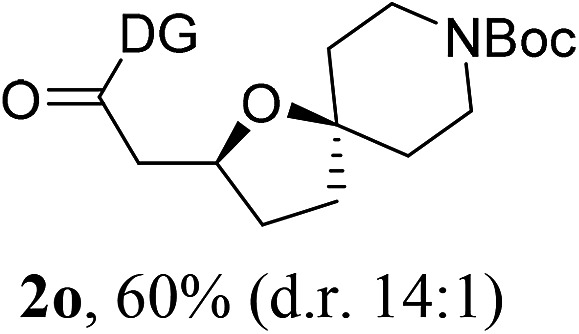
|
|
|
|
|
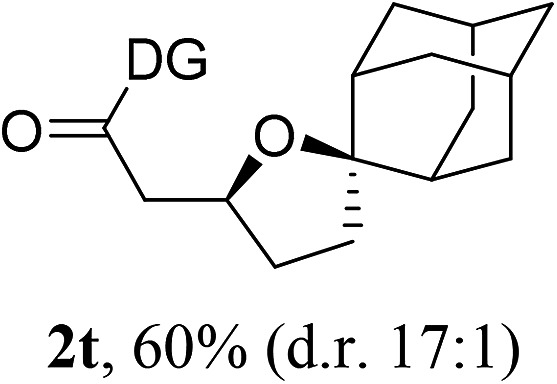
|
|
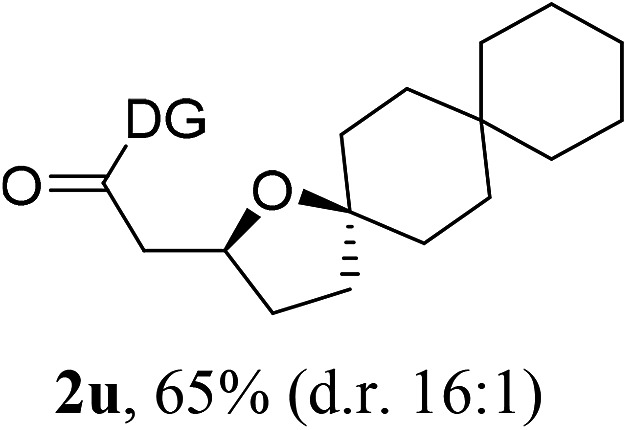
|
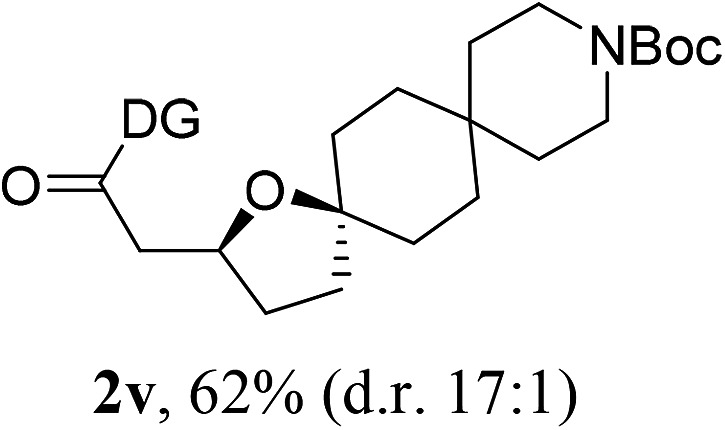
|
|
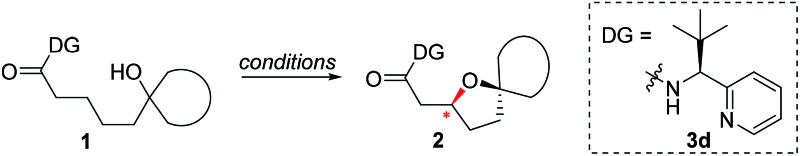
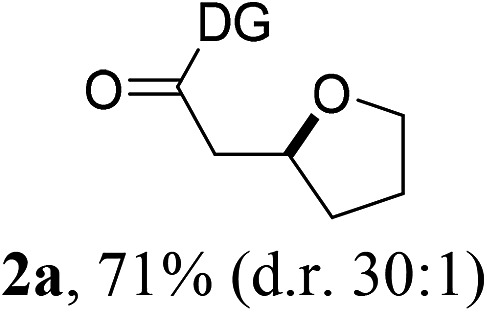
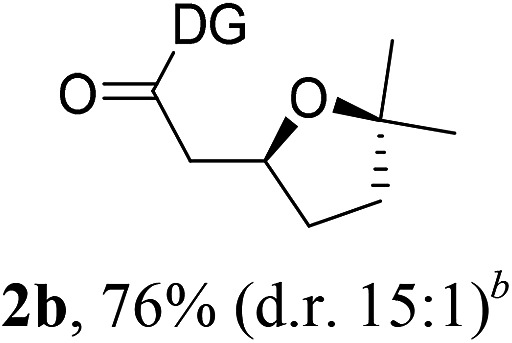
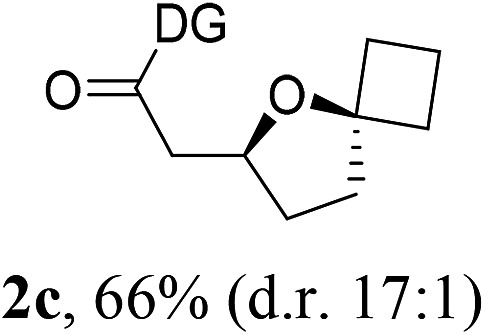
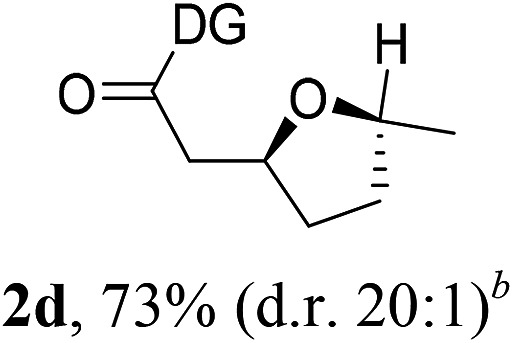
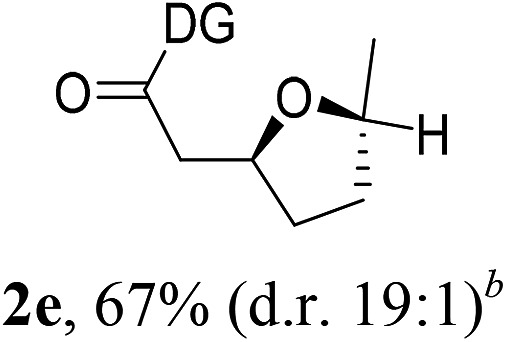
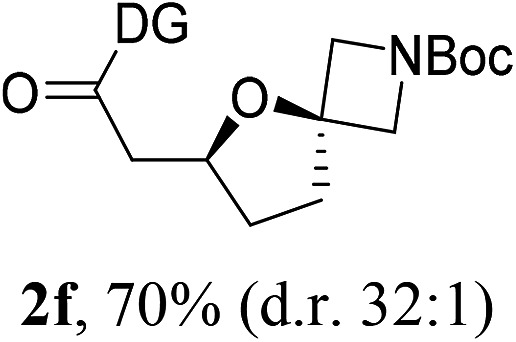
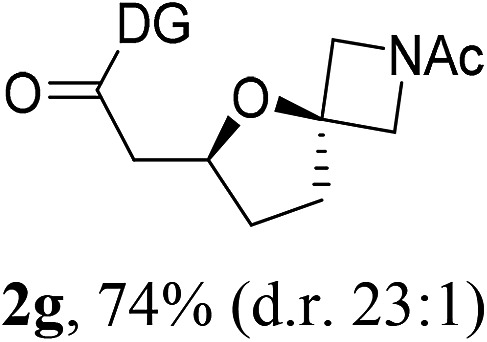
aSubstrate (1.0 equiv.), Pd(OAc)2 (10 mol%), PhI(OAc)2 (3.0 equiv.), and AcOH (4.0 equiv.) in PhMe + EtOH (10 : 1) at 120 °C for 6–18 h. Isolated yields of products. The diastereoisomeric ratio (d.r.) was determined by HPLC analysis.
bThe d.r. was determined by 1H NMR analysis.
cAcOH (8.0 equiv.) was used.
