Abstract
Aim:
The body mass index (BMI), the most used anthropometric index of obesity, has an important limitation, not taking into consideration the distribution of body fat. We developed a new simple index: the waist-corrected BMI (wBMI), calculated as waist circumference (WC) × BMI. The study aim was to assess the role of wBMI, compared to BMI, WC, and Waist-to-Height Ratio (WHtR) in predicting abnormal cardiac geometry, insulin resistance, increased arterial stiffness, and dyslipidemia.
Methods:
This was a cross-sectional study that included 805 patients referred to our Department of Preventive Cardiology for risk factors evaluation and treatment. Eleven echographic and laboratory parameters were determined, and receiver operating characteristic (ROC) curves were derived. Areas under ROC curves (AUC) were used to assess the accuracy of the four indexes to identify unfavorable characteristics.
Results:
There were 29% overweight, 59% obese, and 77% hypertensive patients. Of 11 echographic and laboratory parameters, wBMI, BMI, WHtR, and WC had the largest AUC for identifying 3, 1, 6, and 1 parameters, respectively, but with overlapping 95% confidence intervals. wBMI had the largest AUC for increased arterial stiffness and insulin resistance; also, it was superior to BMI for increased left atrial volume, relative wall thickness, and triglyceride level.
Conclusions:
In a large population with a high prevalence of obesity and hypertension, all four indexes were associated with unfavorable characteristics. wBMI has the theoretical advantage of taking into account simultaneously the global fat mass and distribution and might be useful for a better cardiovascular risk assessment.
Keywords: Cardiac remodeling, index of obesity, obesity
INTRODUCTION
Obesity has become a global epidemic, with an increasing prevalence in all populations and age groups worldwide. Excess body weight is an independent cardiovascular risk factor and is associated with reduced overall survival.[1]
Body mass index (BMI) is the most often used parameter to define the degrees of overweight and obesity, both in epidemiologic studies and by public health organizations. Although this index is simple to calculate and accurate in identifying increased cardiovascular risk, its main limitation is that it evaluates “total obesity,” without accounting for the distribution of body fat. The adverse role of abdominal obesity in the development of cardiovascular diseases (CVD) is well known, and anthropometric parameters for central obesity might refine the evaluation of associated risk.[2] Waist circumference (WC) is correlated with intra-abdominal fat mass, which is an independent risk factor for insulin resistance, diabetes, and CVD.[1,3] On the other hand, WC has several important limitations, as it does not distinguish between subcutaneous and visceral adiposity and is influenced by body size.
To overcome the limitations of BMI, several indexes of obesity have been previously proposed: Matrices that combine BMI and WC, Waist-to-Height Ratio (WHtR), Waist-to-Hip Ratio (WHR), Body Adiposity Index, A body shape index. Nevertheless, there are contradictory data in the literature regarding their role in determining cardiovascular risk beyond BMI.[4]
We developed a new, simple index that takes into account both BMI (correlated with total body fat mass) and WC (reflecting mainly abdominal obesity): waist-corrected BMI (wBMI). It is calculated as WC in meters × BMI in kg/m2, resulting in kg/m. The aim of the study was to assess the role of wBMI, compared to the classical index (BMI) and to other indexes of obesity (WC and WHtR) in predicting abnormal cardiac geometry, insulin resistance, increased arterial stiffness and dyslipidemia in a large group of patients (pts) without the known cardiac disease.
METHODS
Subjects
The study population included 805 consecutive patients referred to our Department of Preventive Cardiology for risk factors evaluation and treatment. Medical history was obtained through direct questioning and by reviewing the medical records.
Anthropometric parameters were measured for all patients. Weight and height were measured in orthostatism, patients wearing only minimal clothing and no footwear, using a calibrated digital weight scale and a standard stadiometer. BMI was determined as weight (kg) divided to squared height (m2). WC was measured using an inelastic tape measure at the midpoint between the lower border of the rib cage and the anterior superior iliac crest in standing position. The World Health Organization (WHO) cutoff values for BMI and WC were used to classify the patients.[5]
Blood pressure (BP) was measured for all patients and hypertension was defined as systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg (at two separate measurements) or use of chronic treatment for systemic arterial hypertension.
All patients were examined using transthoracic echocardiography. The exclusion criteria were: more than mild valvular heart disease, left ventricular ejection fraction ≤50%, or known coronary artery disease.
All patients signed informed consent for participation in this research.
Echocardiography
All measurements were performed according to the American Society of Echocardiography and the European Association of Cardiovascular Imaging recommendations.[6] Left ventricular mass (LVM) was determined using the cube formula: LVM = 0.8 × 1.04 × ([IVS + LVID + PWT]3 − LVID3) + 0.6 g, where IVS is the interventricular septum, LVID is the LV internal diameter, and PWT is the inferolateral wall thickness. Relative wall thickness (RWT) was calculated with the formula (IVS + PWT)/LVID.
Using pulsed wave Doppler echocardiography and pulsed wave tissue Doppler imaging we measured mitral peak E-wave velocity, septal mitral annular e’ velocity, and the E/e’ ratio was calculated.
In the context of a high prevalence of obesity in our study population, we did not use the usual indexation to body surface area of the echocardiographic measurements because this might lead to overcorrection of this parameters.[7] LV diastolic diameter was indexed to height, with a cutoff value of 3.2 cm/m for women and 3.3 cm/m for men according to the reported values in the literature.[8] LVM was indexed to height2.7 as previously described, with a cutoff value of 44 g/m2.7 in women and 49 g/m2.7 for men.[9] LV diastolic volume was indexed to height2.7, and because there are no established cutoff values in the literature for this indexing method, we used an upper-limit value corresponding to the fourth quartile (Q4) in our population. The left atrial (LA) volume was indexed to height, using a cutoff value of 0.333 ml/cm for women and 0.390 ml/cm for men as reported in a previous epidemiological study.[10]
Vascular ultrasound
Carotid intima-media thickness (IMT) was measured using two-dimensional vascular ultrasound, as the distance between lumen-intima and media-adventitia interfaces. The values from the left and right common carotid arteries were averaged. To account for the wide range of ages in our population (18–84 years), in the context of increasing IMT values with age (reported values of 0.007 mm/year),[11] we indexed this parameter to age. The upper-limit was defined as corresponding to Q4.
Beta stiffness index (BSI) was calculated using the formula: BSI = ln (SBP/DBP) × Dd/Ds-Dd, where SBP is systolic BP, DBP is diastolic BP, Ds and Dd are the maximum and minimum common carotid diameters.[12] To determine the common carotid arteries diameters, a color Doppler echocardiography machine was used (Prosound Alfa 10; Aloka, Tokio, Japan), with a 10-MHz linear array probe, implemented with a high-resolution echo-tracking system allowing accurate measurements.
Laboratory parameters
For all patients, blood samples had been collected after >12 h of fasting. Blood glucose and the lipid profile: Total cholesterol, high-density lipoprotein (HDL)-cholesterol, low-density lipoprotein-cholesterol, and triglycerides (TG) were determined. The cutoff values were in accordance with those recommended by current dyslipidemia guidelines.[13] Patients were categorized as dyslipidemic when they had abnormal lipid values or were receiving treatment for dyslipidemia.
For 417 patients, we determined fasting insulinemia and calculated homeostasis model assessment for insulin resistance (HOMA-IR) using the formula: HOMA-IR = (glucose [nmol/L] × insulin [μU/L]/22.5). The cutoff value used was 2.5 which is demonstrated to have good sensitivity and specificity.[14]
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics Version 20 (IBM Corporation, Armonk, New York, United States of America). Descriptive statistics were summarized using means and standard deviation for continuous variables, while categorical variables were presented as numbers and percentages. Receiver operating characteristic (ROC) analyses were used to calculate the area areas under ROC curves (AUC) to assess the accuracy of wBMI, BMI, WHtR, and WC to identify patients with unfavorable characteristics. P < 0.05 was considered to indicate statistical significance.
RESULTS
The main clinical and paraclinical characteristics of the study population are summarized in Table 1. The mean age was 57 ± 14 years, and 44% were men. There were 796 (99%) patients in sinus rhythm, and 9 (1%) patients had atrial fibrillation. The prevalence of overweight and obesity was 29% and 59%, respectively. The prevalence of obesity categories was different across genders (P < 0.001): women had in a higher proportion normal weight (13.9% women vs. 8.8% men, P < 0.05) and obesity Grade III (20.4% in women, 11.6% for men, P < 0.05), while men were more often overweight (34.6% men vs. 24.3% women, P < 0.05) and obese Grade I (30% vs. 21.2%, P < 0.05). In addition, women had more often substantially increased WC according to the WHO categories (86.1% women vs. 66.6% men, P < 0.05). About 68% of the population had dyslipidemia, but only 16% were receiving a statin. There was a high prevalence of systemic arterial hypertension, the majority of patients being treated with antihypertensive drugs (84% of the hypertensive patients).
Table 1.
The main clinical and echographic characteristics of the study population
| Characteristics | Value | |
|---|---|---|
| Clinical characteristics | ||
| Gender-men, n (%) | 353 (44) | |
| Age (years) | 57±14 | |
| Height (cm) | 165±10 | |
| Weight (kg) | 91±23 | |
| BMI (kg/m2) | 33±7 | |
| Body surface area (m2) | 2±0.3 | |
| Overweight, n (%) | 232 (29) | |
| Obesity, n (%) | ||
| Grade I | 202 (25) | |
| Grade II | 144 (18) | |
| Grade III | 133 (16) | |
| Waist-corrected BMI (kg/m) | 36±13 | |
| Waist circumference (cm) | 106±15 | |
| Waist-to-height ratio | 0.64±0.1 | |
| Dyslipidemia, n (%) | 548 (68) | |
| Increased LDL-C, n (%) | 255 (32) | |
| Low-HDL-C, n (%) | 220 (27) | |
| Increased triglycerides | 299 (37) | |
| Treatment, n (%) | ||
| Statin | 130 (16) | |
| ACEI | 200 (25) | |
| ARB | 224 (28) | |
| BB | 231 (29) | |
| CCB | 108 (13) | |
| Diuretic | 273 (34) | |
| Systolic blood pressure (mmHg) | 146±19 | |
| Diastolic blood pressure (mmHg) | 87±10 | |
| Systemic arterial hypertension, n (%) | 622 (77) | |
| Diabetes mellitus, n (%) | 123 (15) | |
| Renal chronic disease, n (%) | 154 (19) | |
| History of smoking, n (%) | 280 (35) | |
| Ecographic characteristics | ||
| LV diastolic diameter (mm) | 48±4 | |
| LV systolic diameter (mm) | 30±4 | |
| LV diastolic diameter/height (mm/m) | 29±2 | |
| LV end-diastolic volume (ml) | 112±24 | |
| LV end-systolic volume (ml) | 37±13 | |
| Interventricular septum (mm) | 12±1 | |
| Infero-lateral wall (mm) | 11±1 | |
| RWT | 0.47±0.6 | |
| LVM (g) | 212±50 | |
| LVMi (g/m2.7) | 54±12 | |
| LV ejection fraction (%) | 62±4 | |
| LA volume (ml) | 41±15 | |
| LA volume/height (ml/m) | 0.24±0.09 | |
| Septal E/E’ ratio | 8.3±2.7 | |
| Beta stiffness index | 7.7±3 | |
| IMT (mm) | 0.9±0.2 | |
ACEI=Angiotensin converting enzyme inhibitors, ARB=Angiotensin receptor blockers, BB=Beta blocker, CCB=Calcium channel blocker, LV=Left ventricle, RWT=Relative wall thickness, LVM=Left ventricular mass, LA=Left atrial, LVMi=LVM index, BMI=Body mass index, LDL-C=Low-density lipoprotein cholesterol, HDL-C=High-density lipoprotein cholesterol
There was a strong correlation between wBMI and BMI (r = 0.975, P < 0.001), WC (r = 0.931, P < 0.001), and WHtR (r = 0.914, P < 0.001).
Figure 1 represents the relationship between BMI and wBMI stratified by gender. By incorporating the information regarding the fat distribution, wBMI will have higher values than BMI in obese patients with WC increased over 1 m, categorizing them into a higher risk class. Using wBMI, 13% of the overweight patients would be reclassified into a higher risk category which would correspond to obesity Grade I, while 27% would be reclassified into a lower risk category. In the same time, almost half of the patients with Grade I obesity (49%) would have even a higher risk than that provided by BMI, given the increased central fat mass, while in 7% of cases BMI may overestimate the health risk. In Figure 1, it can be observed that the trend line for wBMI values in men has a higher slope than the trend line for women, which means that men have higher wBMI values when compared to the corresponding BMI, reflecting the well-known gender influence in fat distribution.
Figure 1.
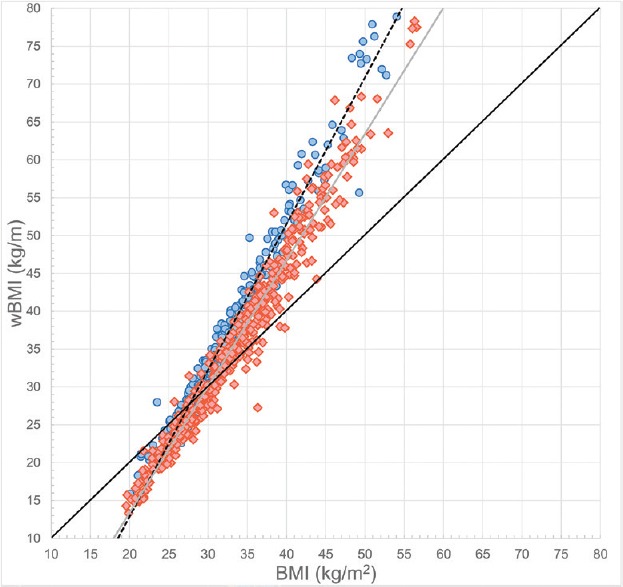
The relationship between body mass index and waist-corrected body mass index stratified by gender. Men and women are represented by blue dots and red diamonds, respectively. The black continuous line represents the mathematical equation f(x) = x. The black dotted and gray lines represent the trend lines for men and women, respectively
Table 1 summarizes the main echocardiographic parameters evaluated in the study population. The majority of patients had increased LVM indexed to height2.7( 74%) and RWT (82%). LV diastolic diameter indexed to height was increased in 7% of cases, and 9% of patients had increased LA volume indexed to height.
ROC curves were derived to evaluate the power of BMI, wBMI, WC, and WHtR to identify patients with unfavorable cardiac remodeling, insulin resistance, increased arterial stiffness, TG levels, and low-HDL-cholesterol levels [Figures 2 and 3].
Figure 2.
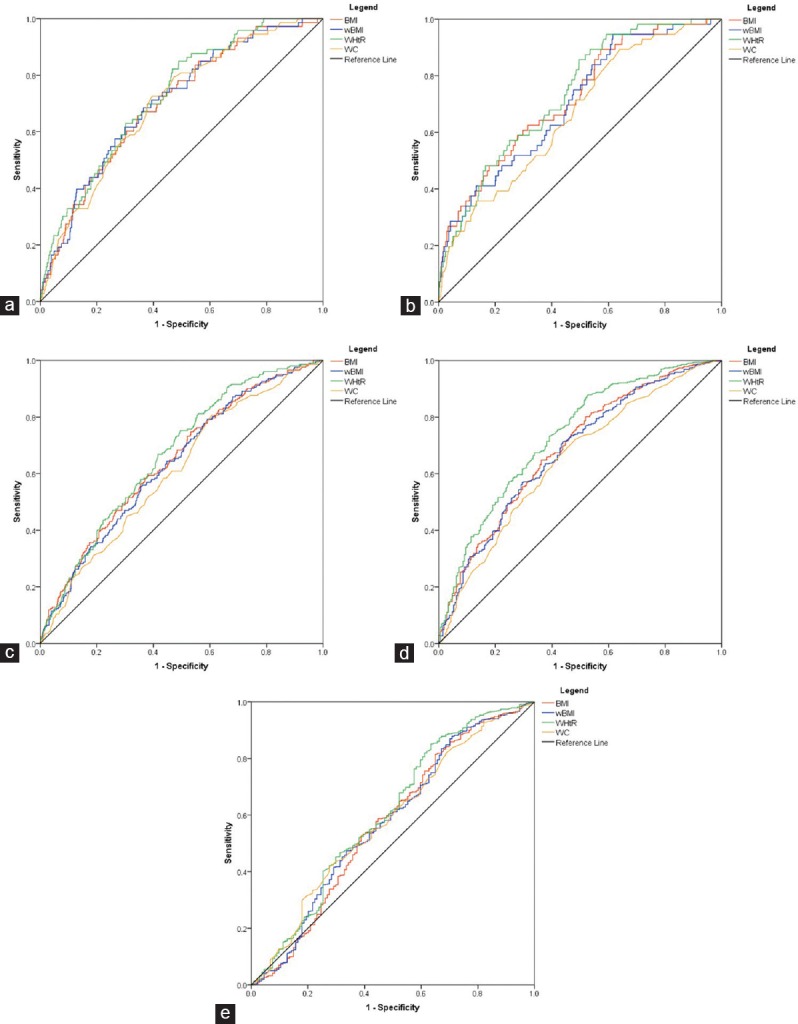
Receiver operating characteristics curves for the four anthropometric indexes and different echocardiographic parameters. (a) Left atrial volume indexed to height; (b) left ventricular diameter indexed to height; (c) left ventricular volume indexed to height2.7; (d) left ventricular mass indexed to height2.7; (e) relative wall thickness
Figure 3.
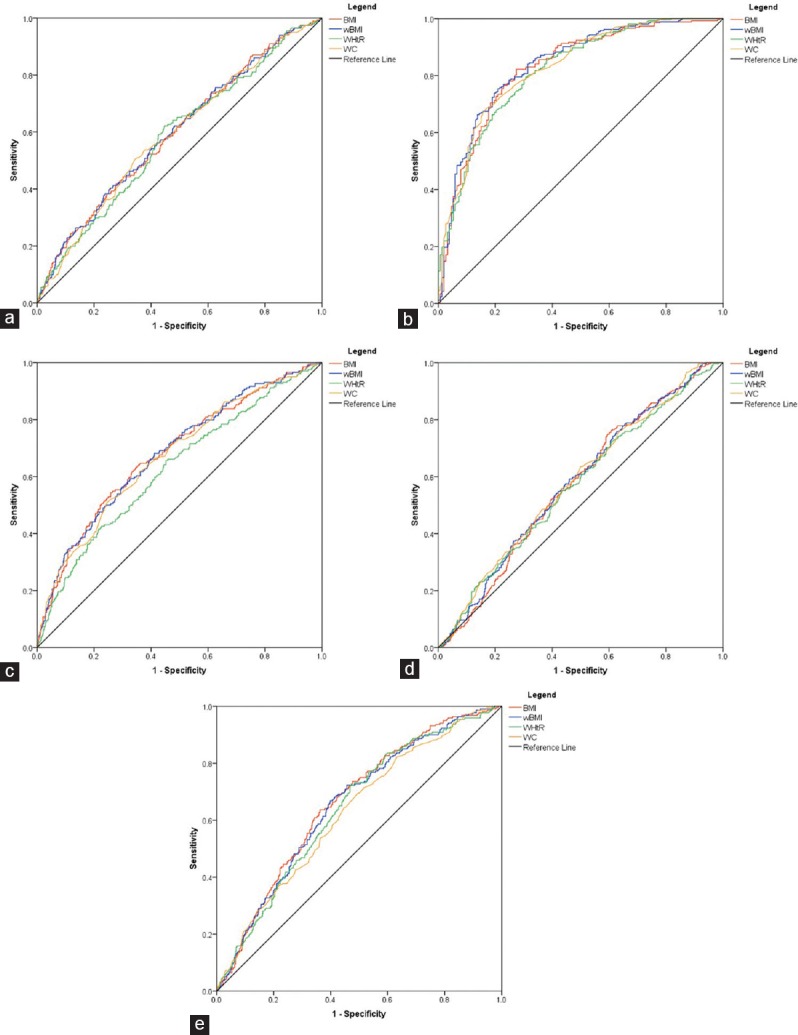
Receiver operating characteristics curves for the four anthropometric indexes and different carotid ultrasound and laboratory parameters. (a) Beta stiffness index; (b) homeostasis model assessment for insulin resistance (c) carotid intima-media thickness indexed to age; (d) triglycerides levels; (e) high-density lipoprotein cholesterol
All four indexes of obesity were correlated with unfavorable cardiac remodeling. wBMI showed a greater AUC than BMI for LA volume and RWT. BMI had a larger AUC than wBMI for LV diameter, LV volume, and LVM. WHtR showed the largest AUC for all these parameters, while WC consistently had the lowest AUC, although the 95% confidence intervals were overlapping between these four indexes [Table 2]. All four parameters had relatively low AUC for identifying E/E’ >8.
Table 2.
Comparison of area under the curve and 95% confidence intervals of anthropometric parameters
| wBMI | BMI | WC | WHtR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC | 95% CI | P | AUC | 95% CI | P | AUC | 95% CI | P | AUC | 95% CI | P | |
| LAVI | 0.71 | 0.65-0.77 | <0.001 | 0.70 | 0.64-0.76 | <0.001 | 0.70 | 0.64-0.76 | <0.001 | 0.73 | 0.67-0.78 | <0.001 |
| LVEDDi | 0.70 | 0.63-0.77 | <0.001 | 0.72 | 0.65-0.79 | <0.001 | 0.66 | 0.59-0.74 | <0.001 | 0.73 | 0.67-0.80 | <0.001 |
| LVEDVi | 0.64 | 0.59-0.68 | <0.001 | 0.65 | 0.60-0.70 | <0.001 | 0.61 | 0.56-0.65 | <0.001 | 0.67 | 0.63-0.71 | <0.001 |
| LVMi | 0.68 | 0.64-0.72 | <0.001 | 0.69 | 0.65-0.73 | <0.001 | 0.65 | 0.60-0.69 | <0.001 | 0.73 | 0.69-0.77 | <0.001 |
| RWT | 0.58 | 0.52-0.63 | 0.006 | 0.57 | 0.51-0.63 | 0.010 | 0.57 | 0.52-0.63 | 0.003 | 0.60 | 0.55-0.66 | <0.001 |
| E/E` >8 | 0.54 | 0.50-0.58 | 0.043 | 0.55 | 0.51-0.59 | 0.025 | 0.54 | 0.49-0.58 | 0.096 | 0.55 | 0.51-0.59 | 0.014 |
| BSI | 0.60 | 0.55-0.64 | <0.001 | 0.60 | 0.55-0.64 | <0.001 | 0.59 | 0.55-0.64 | <0.001 | 0.58 | 0.54-0.63 | <0.001 |
| HOMA-IR | 0.84 | 0.80-0.88 | <0.001 | 0.83 | 0.79-0.87 | <0.001 | 0.83 | 0.79-0.87 | <0.001 | 0.82 | 0.78-0.87 | <0.001 |
| IMT | 0.68 | 0.64-0.72 | <0.001 | 0.68 | 0.64-0.72 | <0.001 | 0.67 | 0.63-0.72 | <0.001 | 0.63 | 0.58-0.67 | <0.001 |
| TG | 0.58 | 0.54-0.62 | <0.001 | 0.57 | 0.53-0.61 | 0.001 | 0.58 | 0.54-0.62 | <0.001 | 0.57 | 0.53-0.61 | 0.001 |
| HDL-C | 0.65 | 0.61-0.69 | <0.001 | 0.66 | 0.62-0.70 | <0.001 | 0.63 | 0.59-0.67 | <0.001 | 0.64 | 0.60-0.68 | <0.001 |
AUC=Area under the curve, BMI=Body mass index, BSI=Beta stiffness index, CI=Confidence intervals, HDL-C=High density lipoprotein cholesterol, HOMA-IR=Homeostasis model assessment for insuline resistance, IMT== Intima-media thickness, LAVI=Left atrial volume indexed to height, LV=Left ventricle, LVEDDi=Left ventricular end-diastolic diameter indexed to height, LVEDVi=Left ventricular end-diastolic volume indexed to height2.7, RWT=Relative wall thickness, TG=Triglycerides, wBMI=Waist corrected BMI, WC=Waist circumference, WHtR=Waist to height ratio, LVMi=LVM index, LVM=Left ventricular mass
All the studied indexes of obesity showed a good correlation with metabolic abnormalities. wBMI had the largest AUC for identifying increased BSI and IMT, followed in decreasing order by BMI, WC, and WHtR. WC had the largest AUC for TG levels, followed by wBMI, BMI, and WHtR, which contrasts with the results obtained for HDL-cholesterol levels: WC had the lowest AUC in identifying patients with low levels of HDL-cholesterol. Furthermore, WHtR was inferior to BMI and wBMI in identifying patients with low-HDL-cholesterol. Again, the 95% confidence intervals were overlapping between these four indexes [Table 2].
In the subgroup of 417 patients, where HOMA-IR was available, all four indexes showed a very good accuracy in identifying patients with insulin resistance, wBMI having the largest AUC, followed by WC, BMI, and WHtR.
DISCUSSION
Obesity is a major public health problem, with a dramatic increase in the global prevalence over the last decades.[5] It is estimated that over 50% of the European Union adult population is overweight, with an overall obesity prevalence of 15.9%.[15]
Excess body weight is associated with several conditions (including ischemic heart disease, stroke, hypertension, diabetes mellitus, cancer, infertility, sleep apnea, osteoarthritis, gout, and dementia) being one of the most important modifiable risk factors.[1,5,16]
BMI is correlated with total body fat mass, but it does not provide information about fat distribution and overestimates fat mass in highly muscular adults. Furthermore, BMI underestimates adiposity in the elderly.[17] In the general population, obesity is an independent risk factor for developing heart disease, but among patients with established coronary artery disease or heart failure, it was recognized that obesity (as described by BMI) is associated with lower mortality (the “obesity paradox”).[18] The obesity paradox might be reflective of the BMI limitations in characterizing fat mass and distribution, and the concomitant utilization of central obesity indexes might be useful.[19,20]
Excess abdominal fat is an independent risk factor for insulin resistance, diabetes, adverse lipid profile and CVD,[1,3] so similar BMI values may not correspond to the same associated health risk in different individuals. Recently, it was recognized the role of so-called normal weight obesity (i.e., normal body weight by BMI, but with high-fat percentage) as a cardiovascular risk factor.[21] It was shown that using BMI as the sole anthropometric index of obesity would misclassify 25% of patients as having low-cardiovascular risk based on normal BMI.[22] In our study, using wBMI would reclassify 9% of normal and overweight patients in a higher risk category corresponding to obesity Grade I. This might better identify increased cardiovascular risk in nonobese population as characterized by BMI.
WC is a simple and practical index of intra-abdominal fat mass, but it is highly influenced by body size, and it does not distinguish between subcutaneous and visceral adiposity. The metabolic syndrome is strongly correlated with visceral fat accumulation. The association between elevated WC and TG levels is considered a marker of “dysfunctional adipose tissue” (visceral obesity) with an incapacity of storing energy surplus in subcutaneous adipose tissue.[23,24] In our study, WC showed the largest AUC in identifying patients with increased TG levels. Increased values of HOMA-IR, which is an index widely used to detect insulin resistance,[25] were best identified by wBMI. BMI had the largest AUC in identifying low-HDL levels.
To overcome BMI and WC limitations, matrices that combine these two parameters were developed to better estimate the cardiovascular risk owing to the added value of WC in describing the abdominal fat,[26] but this might be a more time-consuming process in daily clinical practice. At the same time, it was reported that WHtR might have a better correlation with increased cardiometabolic risk factors (abnormal HDLc, TC/HDLc ratio, TG, and systolic BP) than this matrix.[22] In this study, WHtR had lower AUC than wBMI in identifying patients with increased TG, insulin resistance and low-HDL-cholesterol levels.
Krakauer and Krakauer proposed A body shape index (ABSI) as WC corrected for weight and height to diminish the influence of BMI over WC and to better estimate the intra-abdominal fat.[27] ABSI is calculated using WC divided into BMI2/3 and height1/2, and it was proved as a risk factor for premature mortality. This index expresses the excess risk from high WC for a given BMI,[27] but it is relatively difficult to calculate, and it must be used in conjunction with BMI.
Obesity, through its specific neurohormonal and metabolic alterations, can lead to hemodynamic changes causing modifications in cardiac and vascular morphology and function. The degree of adiposity is associated with adverse cardiac remodeling, which ultimately leads to the development of so-called obesity cardiomyopathy. It was shown that BMI and WC are associated with a greater LVM, LV volume, concentric remodeling and increased LA dimensions.[3,28,29] These cardiac alterations continue to worsen in obese Grade III patients with increasing BMI values.[30] In addition, a greater duration of obesity was associated with increased LVM.[29] Increased LVM and concentric LV remodeling were proven as important independent risk factors for cardiovascular events.[31,32] Obese patients had increased RWT even after adjusting for hypertension.[3] At the same time, LV diastolic function worsens with increased fat mass.[29,33] Importantly, weight loss is capable of inducing cardiac reverse-remodeling.[3,34] Our data showed that WHtR had the highest AUC when we analyzed LA volume, LV diameter, LV volume, LVM and RWT, although the 95% confidence intervals were overlapping between the four studied indexes. wBMI had higher AUC than BMI for 2 out of 5 echocardiographic parameters of adverse cardiac geometry (LA volume and RWT), while WC had the lowest AUC for all 5 parameters.
Obese patients have higher IMT and arterial stiffness than normal weight patients. It was reported that parameters of central obesity (WC and WHtR) had a better correlation with aortic stiffness than BMI.[34] BSI is a marker for arterial stiffness, detecting subclinical atherosclerosis changes in the vessel wall, and it estimates the arterial compliance independent of the BP effect.[12] Our data showed that wBMI had the largest AUC in predicting increased BSI. In addition, wBMI was slightly superior to the other three indexes in identifying patients with increased IMT.
Although there is a significant correlation between BMI, WC, WHtR, and WHR, and all these parameters demonstrated significant prognostic value, it is still unclear which index of obesity describes better the individual risk, with contradictory data in the literature.[22,35,36] A meta-analysis of 58 prospective studies showed that BMI, WC, and WHR had a similar association with risk of first-onset CVD.[37] These results are different from that reported in INTERHEART, which showed that BMI was weakly associated with myocardial infarction, while WHR ratio was 3 times more strongly related to this outcome.[38] Other authors reported that WC was significantly related to all-cause mortality, even when it was stratified by BMI category.[35,36] In another study, it was shown that WC and WHtR had a better correlation with hypertension, hyperglycemia, and dyslipidemia than BMI. The investigators concluded that the combination of indexes of general and central adiposity would result in a better estimation of CVD risk.[4] Furthermore, a large prospective study showed that adjustment for BMI increased the strength of the association between WC and mortality.[39] On the other hand, other authors reported that the prediction of intra-abdominal fat mass by WC was not improved by the addition of BMI.[40] In our study, of 11 echographic and laboratory parameters, wBMI, BMI, WHtR, and WC had the largest AUC for identifying 3, 1, 6, and 1 parameters, respectively, but with relatively small differences and overlapping 95% confidence intervals.
Study limitations
Given the enrolling method, the prevalence of multiple associated cardiovascular risk factors was high in our study, and therefore, the results are not powerful enough to be applied to the general overweight and obese population. Because of the study design, we cannot establish a temporal sequence of the observed cardiovascular abnormalities. This was a cross-sectional study, which did not allow the assessment of obesity duration, a factor which might influence the studied parameters. Participants were receiving different classes of antihypertensive medication, and this could be a confounding factor for which we did not account for in our analysis.
CONCLUSIONS
Combining BMI and WC into a single index might be useful for a better characterization of obesity regarding the cardiovascular risk. Theoretically, wBMI has the advantage of taking into account simultaneously the global fat mass and fat distribution and therefore, could overcome some limitations of BMI and WC. Using this index, 47% of the overweight and obese Grade I patients would be reclassified in lower or higher risk categories, secondary to the additional prognostic value of WC. In a large population with a high prevalence of obesity and hypertension, wBMI, BMI, WC, and WHtR were all associated with adverse cardiac remodeling patterns, increased arterial stiffness, increased insulin resistance, and unfavorable lipid profile. Although wBMI showed the largest AUC for BSI, IMT, and HOMA-IR, and was superior to BMI in identifying increased LA volume, RWT, and TG levels, the differences were small, with overlapping 95% confidence intervals.
Given the known prognostic implications of these structural and functional abnormalities, it is likely that wBMI might be useful for a better stratification of cardiovascular risk. Further studies are needed to specifically address the prognostic role of this new index compared to the conventional ones.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wellington: New Zealand Health Survey; 2015. New Zealand Ministry of Health. Understanding Excess Body Weight. [Google Scholar]
- 2.Guasch-Ferré M, Bulló M, Martínez-González MÁ, Corella D, Estruch R, Covas MI, et al. Waist-to-height ratio and cardiovascular risk factors in elderly individuals at high cardiovascular risk. PLoS One. 2012;7:e43275. doi: 10.1371/journal.pone.0043275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alpert MA, Omran J, Mehra A, Ardhanari S. Impact of obesity and weight loss on cardiac performance and morphology in adults. Prog Cardiovasc Dis. 2014;56:391–400. doi: 10.1016/j.pcad.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Lam BC, Koh GC, Chen C, Wong MT, Fallows SJ. Comparison of body mass index (BMI), body adiposity index (BAI), waist circumference (WC), waist-to-hip ratio (WHR) and waist-to-height ratio (WHtR) as predictors of cardiovascular disease risk factors in an adult population in Singapore. PLoS One. 2015;10:e0122985. doi: 10.1371/journal.pone.0122985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i. [PubMed] [Google Scholar]
- 6.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–70. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 7.Zong P, Zhang L, Shaban NM, Peña J, Jiang L, Taub CC, et al. Left heart chamber quantification in obese patients: How does larger body size affect echocardiographic measurements? J Am Soc Echocardiogr. 2014;27:1267–74. doi: 10.1016/j.echo.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Ilercil A, O’Grady MJ, Roman MJ, Paranicas M, Lee ET, Welty TK, et al. Reference values for echocardiographic measurements in urban and rural populations of differing ethnicity: The strong heart study. J Am Soc Echocardiogr. 2001;14:601–11. doi: 10.1067/mje.2001.113258. [DOI] [PubMed] [Google Scholar]
- 10.Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM, et al. Left atrial volume as an index of left atrial size: A population-based study. J Am Coll Cardiol. 2003;41:1036–43. doi: 10.1016/s0735-1097(02)02981-9. [DOI] [PubMed] [Google Scholar]
- 11.Polak JF, Pencina MJ, Meisner A, Pencina KM, Brown LS, Wolf PA, et al. Associations of carotid artery intima-media thickness (IMT) with risk factors and prevalent cardiovascular disease: Comparison of mean common carotid artery IMT with maximum internal carotid artery IMT. J Ultrasound Med. 2010;29:1759–68. doi: 10.7863/jum.2010.29.12.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antonini-Canterin F, Carerj S, Di Bello V, Di Salvo G, La Carrubba S, Vriz O, et al. Arterial stiffness and ventricular stiffness: A couple of diseases or a coupling disease. A review from the cardiologist's point of view? Eur J Echocardiogr. 2009;10:36–43. doi: 10.1093/ejechocard/jen236. [DOI] [PubMed] [Google Scholar]
- 13.Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 14.Salgado AL, Carvalho LD, Oliveira AC, Santos VN, Vieira JG, Parise ER, et al. Insulin resistance index (HOMA-IR) in the differentiation of patients with non-alcoholic fatty liver disease and healthy individuals. Arq Gastroenterol. 2010;47:165–9. doi: 10.1590/s0004-28032010000200009. [DOI] [PubMed] [Google Scholar]
- 15.Eurostat European Health Interview Survey: Almost 1 Adult in 6 in the EU is Considered Obese. Eurostat. 2016. [Last accessed date on 2017 Sep 01]. Available from: http://www.ec.europa.eu/eurostat/documents/2995521/7700898/3-20102016-BP-EN.pdf/c26b037b-d5f3-4c05-89c1.00bf0b98d646 .
- 16.Rosengren A, Skoog I, Gustafson D, Wilhelmsen L. Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med. 2005;165:321–6. doi: 10.1001/archinte.165.3.321. [DOI] [PubMed] [Google Scholar]
- 17.Pasco JA, Nicholson GC, Brennan SL, Kotowicz MA. Prevalence of obesity and the relationship between the body mass index and body fat: Cross-sectional, population-based data. PLoS One. 2012;7:e29580. doi: 10.1371/journal.pone.0029580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 19.Oreopoulos A, Padwal R, Norris CM, Mullen JC, Pretorius V, Kalantar-Zadeh K, et al. Effect of obesity on short-and long-term mortality postcoronary revascularization: A meta-analysis. Obesity (Silver Spring) 2008;16:442–50. doi: 10.1038/oby.2007.36. [DOI] [PubMed] [Google Scholar]
- 20.Bastien M, Poirier P, Lemieux I, Després JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis. 2014;56:369–81. doi: 10.1016/j.pcad.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Oliveros E, Somers VK, Sochor O, Goel K, Lopez-Jimenez F. The concept of normal weight obesity. Prog Cardiovasc Dis. 2014;56:426–33. doi: 10.1016/j.pcad.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Ashwell M, Gibson S. Waist-to-height ratio as an indicator of ‘early health risk’: Simpler and more predictive than using a ‘matrix’ based on BMI and waist circumference. BMJ Open. 2016;6:e010159. doi: 10.1136/bmjopen-2015-010159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemieux I, Pascot A, Couillard C, Lamarche B, Tchernof A, Alméras N, et al. Hypertriglyceridemic waist: A marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation. 2000;102:179–84. doi: 10.1161/01.cir.102.2.179. [DOI] [PubMed] [Google Scholar]
- 24.Lemieux I, Poirier P, Bergeron J, Alméras N, Lamarche B, Cantin B, et al. Hypertriglyceridemic waist: A useful screening phenotype in preventive cardiology? Can J Cardiol. 2007;23(Suppl B):23B–31B. doi: 10.1016/s0828-282x(07)71007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song Y, Manson JE, Tinker L, Howard BV, Kuller LH, Nathan L, et al. Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women: The women's health initiative observational study. Diabetes Care. 2007;30:1747–52. doi: 10.2337/dc07-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Institute for Health and Care Excellence (UK). Identification, Assessment and Management of Overweight and Obesity in Children, Young People and Adults. (Clinical Guideline 189) 2014. [Last accessed on 2017 Aug 15]. Available from: http://www.nice.org.uk/guidance/cg189 . [PubMed]
- 27.Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS One. 2012;7:e39504. doi: 10.1371/journal.pone.0039504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korre M, Porto LG, Farioli A, Yang J, Christiani DC, Christophi CA, et al. Effect of body mass index on left ventricular mass in career male firefighters. Am J Cardiol. 2016;118:1769–73. doi: 10.1016/j.amjcard.2016.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reis JP, Allen N, Gibbs BB, Gidding SS, Lee JM, Lewis CE, et al. Association of the degree of adiposity and duration of obesity with measures of cardiac structure and function: The CARDIA study. Obesity (Silver Spring) 2014;22:2434–40. doi: 10.1002/oby.20865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antonini-Canterin F, Mateescu AD, Vriz O, La Carrubba S, Di Bello V, Carerj S, et al. Cardiac structure and function and insulin resistance in morbidly obese patients: Does superobesity play an additional role? Cardiology. 2014;127:144–51. doi: 10.1159/000355260. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong AC, Gidding S, Gjesdal O, Wu C, Bluemke DA, Lima JA, et al. LV mass assessed by echocardiography and CMR, cardiovascular outcomes, and medical practice. JACC Cardiovasc Imaging. 2012;5:837–48. doi: 10.1016/j.jcmg.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsao CW, Gona PN, Salton CJ, Chuang ML, Levy D, Manning WJ, et al. Left ventricular structure and risk of cardiovascular events: A Framingham heart study cardiac magnetic resonance study. J Am Heart Assoc. 2015;4:e002188. doi: 10.1161/JAHA.115.002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alpert MA, Omran J, Bostick BP. Effects of obesity on cardiovascular hemodynamics, cardiac morphology, and ventricular function. Curr Obes Rep. 2016;5:424–34. doi: 10.1007/s13679-016-0235-6. [DOI] [PubMed] [Google Scholar]
- 34.Antonini-Canterin F, Pellegrinet M, Marinigh R, Favretto G. Role of cardiovascular ultrasound in the evaluation of obese subjects. J Cardiovasc Echogr. 2014;24:67–71. doi: 10.4103/2211-4122.143961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Staiano AE, Reeder BA, Elliott S, Joffres MR, Pahwa P, Kirkland SA, et al. Body mass index versus waist circumference as predictors of mortality in Canadian adults. Int J Obes (Lond) 2012;36:1450–4. doi: 10.1038/ijo.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–20. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 37.Emerging Risk Factors Collaboration. Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: Collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–95. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: A case-control study. Lancet. 2005;366:1640–9. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 39.Jacobs EJ, Newton CC, Wang Y, Patel AV, McCullough ML, Campbell PT, et al. Waist circumference and all-cause mortality in a large US cohort. Arch Intern Med. 2010;170:1293–301. doi: 10.1001/archinternmed.2010.201. [DOI] [PubMed] [Google Scholar]
- 40.Berentzen TL, Ängquist L, Kotronen A, Borra R, Yki-Järvinen H, Iozzo P, et al. Waist circumference adjusted for body mass index and intra-abdominal fat mass. PLoS One. 2012;7:e32213. doi: 10.1371/journal.pone.0032213. [DOI] [PMC free article] [PubMed] [Google Scholar]


