Figure 1. Ca2+ handling components in skeletal muscle Excitation-contraction coupling.
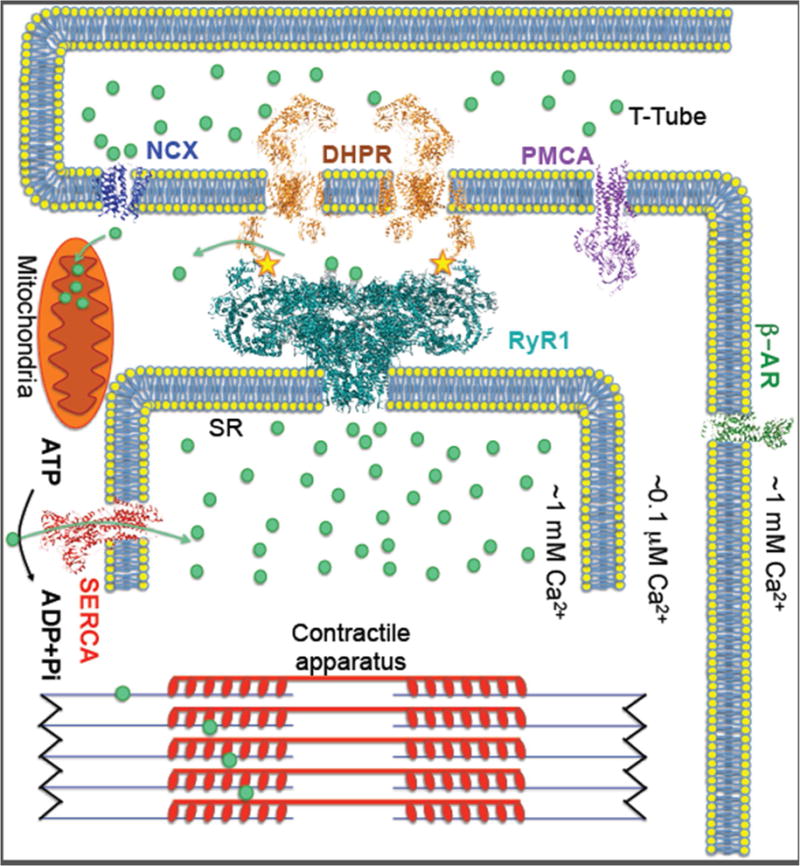
Upon plasma membrane depolarization DHPR (dihydropyridine receptor) mechanically activates RyR1 to release SR Ca2+, which triggers muscle contraction. The Ca2+ ATPase SERCA1a pumps Ca2+ back into the SR and PMCA (plasma membrane Ca2+ ATPase) pump out the Ca2+ from cytoplasm causing muscle relaxation. The RyR1 complex includes: calstabin1, which stabilizes the closed state of the channel, kinases and phosphatases that modulate the channel through phosphorylation (yellow stars marks the phosphorylation site), and calmodulin. RyR1 can be activated by stress pathways via β-adrenergic receptors (β-AR) resulting in increased Ca2+ release and enhanced muscle performance. SR Ca2+ release may also modulate mitochondrial function, particularly during states of chronic stress (e.g. muscular dystrophy), resulting in generation of reactive oxygen species that can oxidize RyR1 and deplete calstabin1 from the channel, rendering the channels leaky. Leaky RyR1 channels were shown to be a contributing factor to diseases including muscle myopathies, Duchenne muscular dystrophy and cancer.
