Figure 6. Depolarizing currents elicited at 100 μM concentration: persistent current defined as A) time constant of recovery, T50, or B) current remaining after removing the compound externally (washout).
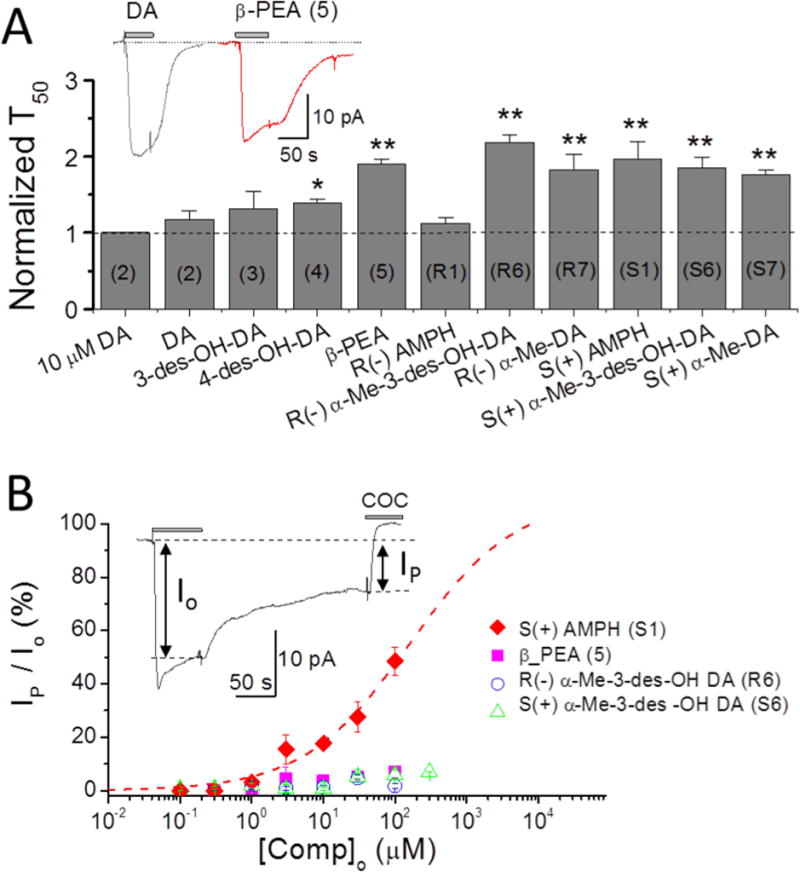
A. Time constants of recovery after removal of test compounds. All agents induce a significantly slower recovery than DA except 3-des-OH DA (3) and R(−)AMPH (R1). Compounds designated with (*) or (**) and with T50 values greater than 1 (dotted line) are referred to as ‘persistent’ at 100 μM; only β-PEA also passes this test at 10 μM (Fig. 3). S(+)AMPH generates a prominent ‘shelf’ with a flattened current, also seen at 10 μM in Fig. 2, while other agents have only delayed recovery times expressed as T50 > 1 at this higher concentration; however, β-PEA may also show a ‘shelf’ or ‘flattening’, as shown in the inset. Data recorded at −60 mV. Note: the numbers for each compound are labeled inside the column. The symbols ** imply p < 0.001 and * p < 0.01.
B. The relative persistent currents of four compounds selected from panel A for slow recovery times are shown here as a function of concentration. The current Io is the induced depolarizing current just before washout, and Ip is the current remaining after washout just before block with cocaine. Only S(+)AMPH (S1) stands out at all concentrations; β-PEA (5), with relatively slow recovery and a ‘shelf’ similar to S(+)AMPH, has a much lower persistent current than S(+)AMPH (S1) by this definition, as do R(−)α-Me 3-des OH DA (R6) and S(+)α-Me-3-des OH (S6) at all concentrations.
