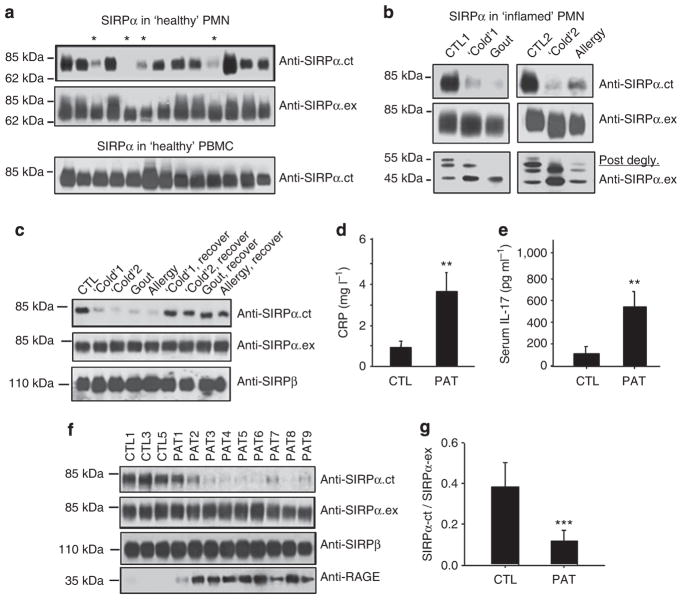
Figure 2. The correlation between the loss of SIRPα ITIMs in PMN and active inflammation.
(a) Peripheral PMNs and PBMCs were isolated from randomly selected ‘healthy’ donors. WB analyses of SIRPα expression by anti-SIRPα.ex and anti-SIRPα.ct antibodies. The samples that had decreased reactivity with anti-SIRPα.ct are marked ‘*’. (b) PMNs obtained from a donor with active gout, two donors with upper respiratory inflammation (‘Cold’) and a donor with upper respiratory allergies were analysed for SIRPα protein. PMNs from two healthy donors were used as controls (CTL1 and CTL2). Protein deglycosylation results were shown in the lower panel. (c) Recovery of anti-SIRPα.ct reactivity in PMNs isolated from the same donors after their ‘cold’, gout or allergy was subsided. (d) Levels of C-reactive protein (CRP) and (e) levels of IL-17 in plasma of healthy donors (CTL, n = 12) and type 2 diabetic patients with cardiovascular complications (PAT, n = 25) detected by enzyme-linked immunosorbent assay. The data presented as means±s.d., **P<0.01 determined by Student’s t-test. (f,g) WB analyses of SIRPα in PMNs of 25 type 2 diabetic patients with cardiovascular complications (PAT) and 12 healthy controls (CTL). RAGE, receptor for advanced glycation end products. Note that the WB images (f) show partial results, whereas the densitometry analysed all PMN samples (g). The data presented as means±s.d., ***P<0.005 determined by Student’s t-test.