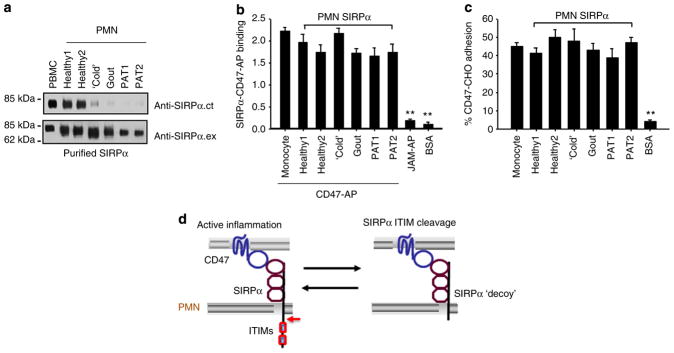
Figure 7. The deletion of intracellular ITIMs does not affect the extracellular binding of SIRPα to CD47.
Various SIRPα proteins, affinity purified from monocytes (PBMCs) and PMNs of healthy donors or donors under various inflammatory conditions, were confirmed as ITIM+SIRPα (from PBMCs or PMNs of healthy donors) or ITIM−SIRPα (from PMNs of inflammatory donors) by WB analyses (a). Purified SIRPα proteins were immobilized onto 96-well microtitre plates and then were incubated with 2 μg ml−1 CD47-AP (b), or CD47-expressing CHO cells (CD47-CHO)6 that were pre-loaded with BCECF (2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein) fluorescence dye (c). After washing, the binding of CD47-AP was assessed by measuring the AP activity. The paralleled binding of JAM-AP1, a chimera containing the extracellular domain of junctional adhesion molecule A (JAM-A), and BSA to immobilized SIRPα served as the non-binding controls. The adhesion of CD47-CHO cells to immobilized SIRPα was determined by measuring cell fluorescence intensity. CD47-CHO cell adhesion to BSA-coated wells served as a negative control. The results (means±s.d.) represent three independent experiments with triplicates in each condition. **P<0.01 assessed by Student’s t-test. (d) Schematic illustration of the positive feedback loop mediated by inflammation-induced cleavage of SIRPα cytoplasmic ITIMs under chronic conditions.