Abstract
Modeling human disease in animals is an important strategy to discover potential methods of intervention. We suggest that there is much to be gained by employing a multi-model approach that takes advantage of different animal systems used in the laboratory simultaneously. We use the example of modeling Alzheimer’s disease in Drosophila melanogaster, Caenorhabditis elegans, and Danio rerio to illustrate how such an approach can be employed to investigate the pathophysiology of the disease.
Keywords: Alzheimer’s disease, CRY2, Drosophila melanogaster, Caenorhabditis elegans, Danio rerio, optogenetics
1. Introduction
Animal models are indispensable tools for understanding biology and for designing strategies to mitigate human disease. An important question to address then is the choice of the animal species when modeling a disorder or a disease. After all, animals may not suffer from the same ailments as humans do. A popular idea in the field of neuroethology is to study a ‘champion’ species or a specialist in the particular motor or sensory function of interest [1,2,3]. An echo-locating bat may reveal the workings of auditory processing or a cephalopod may help us identify a neural mechanism for camouflage. Indeed, unusual insights into cancer pathology have come to us via naked mole rats that are highly resistant to cancers in spite of a life spanning around three decades [4,5]. However, this is not entirely suitable to tease apart the molecular and cellular processes underlying disease progression in many cases that often require extensive experimentation and elaborate screens.
Historically, rodents and non-human primates have received the most attention in medicine as mammalian models, particularly in the final phases of drug or vaccine development prior to clinical trials in humans. However, the costs and infrastructure associated with running such experiments at scale are fast becoming prohibitive. Instead Drosophila melanogaster, Caenorhabditis elegans, and Danio rerio (colloquially, fruit flies, nematode worms, and zebrafish) are increasingly used to study various aspects of diseases for exactly the same reasons that made them popular for studying fundamental biology—the ease of rearing them in the lab, performing genetic manipulations, molecular and biochemical analyses, and behavioral studies. For instance, fruit fly, worm, and zebrafish models have been critical to elucidate the RAS signaling pathway in cancer, polyglutamine-expansion disorders, and melanoma respectively among other diseases [6,7,8,9,10]. We suggest that using all three in combination may circumvent some of the challenges posed in the search of a ‘champion’ animal model that satisfactorily recapitulates a disease and allows studies of genetics, physiology, and behavior. In addition, the tools available for studying development in these systems become highly advantageous for dissociating incidental age-related changes from changes causally linked in an age dependent disease.
2. Alzheimer’s Disease: An Unresolved Problem
In this commentary, we turn our attention to Alzheimer’s disease (AD). AD is the most common cause of dementia, and a debilitating age-associated neurodegenerative disease currently lacking effective treatment [11,12]. According to a non-profit, voluntary organization specializing in Alzheimer’s care, AD is the sixth leading cause of death in America, and there are now 5.5 million Americans living with it [13]. Globally, there are 46.8 million people living with dementia, including AD and this number is expected to increase to reach 131.5 million people by 2050 [14]. Global burden of disease studies suggest that the cost of AD and other dementia-related illness is in the range of USD 250 billion in America, and over USD 600 billion globally [13,15]. Such statistics highlight the prevalence of AD and other dementia-related illnesses and the grave public health challenge that future generations face.
Even after several decades of research, the exact cause of AD is yet to be clearly established. However, two classic neuropathological signs are associated with the disease. Namely, neurofibrillary tangles and extracellular Amyloid-β (Aβ) plaques in the human brain [16,17]. Tangles are thought to be caused by misfolding and hyperphosphorylation of tau proteins normally involved in microtubule stabilization [17]. Plaques, on the other hand, are aggregates of the beta-amyloid peptide—a cleavage product of a protein that is normally expressed throughout the ubiquitously called the amyloid precursor protein (APP) [18].
While both these processes are associated with AD, here, for the purpose of illustration, we only consider the amyloid hypothesis. According to this hypothesis, the accumulation of Aβ in various forms, as plaques or as soluble oligomers cause neuronal damage and the consequent inflammatory response that eventually leads to the deleterious symptoms associated with AD [19]. Evidence underpinning this association comes from familial cases of early-onset AD, which is linked to germline mutations in APP [20] and the presenilin genes involved in cleaving Aβ from APP [21]. The amyloid hypothesis proponents use pathological similarities between familial and sporadic AD as an argument, as well as observations of other age-related diseases such as the myositis-like phenotype caused by Aβ accumulation in transgenic mice overexpressing Aβ in muscle tissue [22].
Despite such evidence, the pathophysiology of Aβ plaques and their relation to AD is not a settled issue. Critics of the amyloid hypothesis point out findings of Aβ plaques in brains of individuals who did not show any symptoms of AD at the time of death. Other unresolved issues including poor correlation between plaque load and cognitive scores and heterogeneity in pathology have called into question the causal association ascribed to Aβ plaques [23]. There has been a shift of focus from plaques to the other potential malfunctions including to role of soluble forms of Aβ oligomerisation that further triggers a downstream cascade [24]. A recent paper also demonstrated that blood-derived Aβ resulted in pathogenic symptoms of AD in mice models, suggesting that Aβ plaques need not originate in the brain to evoke pathological symptoms [25]. Given the current status, whether extracellular Aβ plaques have a detrimental role in AD, or if they have a protective role before other malfunctions accumulate continues to be a central question [26]. Further, even though extracellular plaques are considered a hallmark of AD, consensus on whether problems arise due to Aβ aggregates formed intracellularly in neurons, or from secreted peptides extracellulary (or either), is also lacking [27,28,29].
3. Combining Forces
To unravel these questions, one approach could be to first clearly evaluate if Aβ plaque formation has any impact on neuronal health and cognitive performance. One approach is to use a multi-model study design that capitalizes on the unique advantages of flies, nematodes, and zebrafish.
Research using Drosophila has been vital to dissect genetic pathways critical for the formation, migration, and activity of Aβ plaques [6]. Using the galactose-inducible yeast system, commonly known as the Gal4/UAS system [30], transgenic flies can be easily generated to analyze several genetic pathways in a short period. The genetic pathways identified from such analyses can be further investigated in C. elegans biochemically. C. elegans has been widely employed to investigate the effects of aging and various neurodegenerative diseases, including AD [7]. With a short life cycle of three days from egg to adult, they are highly amenable to evaluate the “druggability” of targets among the biochemical pathways [31]. Feeding assays can be designed to implement large-scale drug screens or screens based on RNA interference (RNAi) knockdown [7]. Another factor when modeling human pathologies is to recapitulate the biological outcomes described in humans. Compared to the two invertebrate animal models, the vertebrate nervous system of zebrafish is more appropriate to study the consequences of Aβ plaques in the brain. Due to their transparent nature, zebrafish are also an excellent tool to investigate neural activity in vivo, thereby making them particularly useful when studying neurophysiology. Using whole brain imaging and cell-type specific labeling techniques, unique neurons in any region of the brain can be studied with ease [32]. In addition, several behavioral paradigms have already been developed that can be employed to evaluate the cognitive impact if neurodegeneration is occurring [33].
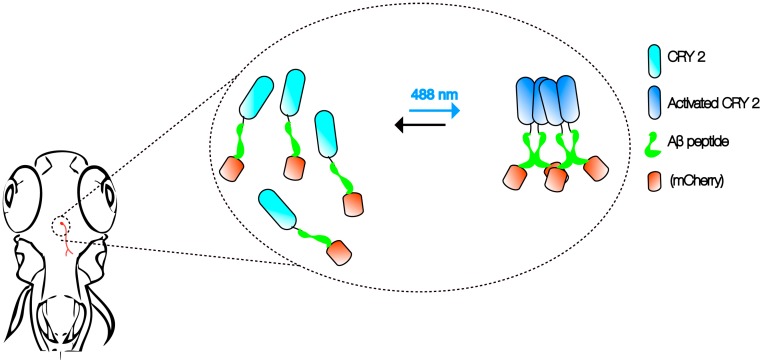
Advancements in imaging and genetic techniques further enhance the utility of this multi-model approach. One such technique is optogenetics—an extremely versatile tool using light to examine physiological processes [34]. Compared to the constraints posed by electrical recordings and pharmacological interventions in the past, optogenetics can be used to examine specific cellular activities with extremely high precision and speed in vivo. Recently, cryptochrome2 (CRY2) protein system from the Arabidopsis was modified as an optogenetic tool to study the control of cortical actin dynamics in cell culture and cell contractility during tissue morphogenesis in Drosophila embryos [35,36]. Activation of the CRY2 oligomerization at 488 nm and inhibition in the dark allows researchers to manipulate protein–protein interactions without the need for other external agents [37].
It is not hard to imagine that the same CRY2 optogenetic system can be employed with Aβ to create an in vivo, light-dependent, oligomerization switch for the formation or dissolution of Aβ aggregates (Figure 1). By generating transgenics in all three organisms in the neurons, or cells of interest, the spatiotemporal kinetics of Aβ plaque formation can be controlled at will to address the question of their relationship and AD pathogenesis. Light-inducible aggregates in worms will allow fast screening for drugs that are effective in dissolution when administered before, or after, or when plaques form repeatedly. Transparency of zebrafish allows imaging the kinetics of plaque formation. We envisage that the use of such a technique in fish will also shed light on the link between intracellular Aβ peptide aggregates and extracellular plaques. In addition, promising drugs screened in the worm studies can be rapidly evaluated in the context of a vertebrate brain. A battery of behavioral assays reporting on learning, memory, anxiety, and depression can then be employed to assess the impact [33]. Finally, flies that express Aβ CRY2 in specific organs, at specific times, will be highly informative to understand the spatiotemporal dynamics of plaque location for any pathogenic effect to be visible, as well as to identify genetic interactors at different stages in case pathogenicity is observed [25]. Together, these will likely provide firm answers to the questions raised by the amyloid hypothesis of AD, especially on the role of soluble vs. Aβ plaques as plaque formation can be engineered externally.
Figure 1.
Aβ CRY2 optogenetic system. The schematic shows an example of in vivo, light-dependent, oligomerization switch for the formation or dissolution of Aβ aggregates in region of interest (neurons) in a transgenic zebrafish.
The potential of using such a combinatory approach of various model organisms and optogenetics is therefore vast, can be applied to other diseases and will only continue to expand as further improvements in technology become available.
Acknowledgments
We thank the two anonymous reviewers and Nicholas Tolwinski for comments. This work was supported by Yale-NUS College through grants R-607-265-225-121 and IG16-LR003; and by Agency for Science, Technology and Research (A*STAR).
Author Contributions
C.H.L and A.S.M wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bullock T.H. Comparative neuroscience holds promise for quiet revolutions. Science. 1984;225:473–478. doi: 10.1126/science.6740319. [DOI] [PubMed] [Google Scholar]
- 2.Bullock T.H. “Simple” model systems need comparative studies: Differences are as important as commonalities. Trends Neurosci. 1986;9:470–472. doi: 10.1016/0166-2236(86)90151-7. [DOI] [Google Scholar]
- 3.Heiligenberg W. The neural basis of behavior: A neuroethological view. Annu. Rev. Neurosci. 1991;14:247–267. doi: 10.1146/annurev.ne.14.030191.001335. [DOI] [PubMed] [Google Scholar]
- 4.Tian X., Azpurua J., Hine C., Vaidya A., Myakishev-Rempel M., Ablaeva J., Mao Z., Nevo E., Gorbunova V., Seluanov A. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature. 2013;499:346. doi: 10.1038/nature12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delaney M.A., Ward J.M., Walsh T.F., Chinnadurai S.K., Kerns K., Kinsel M.J., Treuting P.M. Initial case reports of cancer in naked mole-rats (heterocephalus glaber) Vet. Pathol. 2016;53:691–696. doi: 10.1177/0300985816630796. [DOI] [PubMed] [Google Scholar]
- 6.Pandey U.B., Nichols C.D. Human disease models in drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011;63:411–436. doi: 10.1124/pr.110.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markaki M., Tavernarakis N. Modeling human diseases in caenorhabditis elegans. Biotechnol. J. 2010;5:1261–1276. doi: 10.1002/biot.201000183. [DOI] [PubMed] [Google Scholar]
- 8.Santoriello C., Zon L.I. Hooked! Modeling human disease in zebrafish. J. Clin. Investig. 2012;122:2337–2343. doi: 10.1172/JCI60434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moulton M.J., Letsou A. Modeling congenital disease and inborn errors of development in drosophila melanogaster. Dis. Models Mech. 2016;9:253–269. doi: 10.1242/dmm.023564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kessler M., Rottbauer W., Just S. Recent progress in the use of zebrafish for novel cardiac drug discovery. Expert Opin. Drug Discov. 2015;10:1231–1241. doi: 10.1517/17460441.2015.1078788. [DOI] [PubMed] [Google Scholar]
- 11.Kumar A., Singh A., Ekavali A review on alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015;67:195–203. doi: 10.1016/j.pharep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 12.De-Paula V.J., Radanovic M., Diniz B.S., Forlenza O.V. Alzheimer’s disease. Sub-Cell. Biochem. 2012;65:329–352. doi: 10.1007/978-94-007-5416-4_14. [DOI] [PubMed] [Google Scholar]
- 13.Alzheimer’s Association 2017 Alzheimer’s Disease Facts and Figures. [(accessed on 10 November 2017)]; Available online: https://www.alz.org/documents_custom/2017-facts-and-figures.pdf.
- 14.Alzheimer’s Disease International . World Alzheimer Report 2016: Improving Healthcare for People Living with Dementia. Alzheimer’s Disease International (ADI); London, UK: 2016. [Google Scholar]
- 15.Alzheimers.net 2016 Alzheimer’s Statistics. [(accessed on 10 November 2017)]; Available online: https://www.alzheimers.net/resources/alzheimers-statistics/
- 16.Alexander A.G., Marfil V., Li C. Use of caenorhabditis elegans as a model to study alzheimer’s disease and other neurodegenerative diseases. Front. Genet. 2014;5:279. doi: 10.3389/fgene.2014.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iqbal K., Alonso Adel C., Chen S., Chohan M.O., El-Akkad E., Gong C.X., Khatoon S., Li B., Liu F., Rahman A., et al. Tau pathology in alzheimer disease and other tauopathies. Biochim. Biophys. Acta. 2005;1739:198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Kang J., Lemaire H.G., Unterbeck A., Salbaum J.M., Masters C.L., Grzeschik K.H., Multhaup G., Beyreuther K., Muller-Hill B. The precursor of alzheimer’s disease amyloid a4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 19.Eskelinen M.H., Kivipelto M. Caffeine as a protective factor in dementia and alzheimer’s disease. J. Alzheimer’s Dis. JAD. 2010;20(Suppl. 1):S167–S174. doi: 10.3233/JAD-2010-1404. [DOI] [PubMed] [Google Scholar]
- 20.Chartier-Harlin M.C., Crawford F., Houlden H., Warren A., Hughes D., Fidani L., Goate A., Rossor M., Roques P., Hardy J., et al. Early-onset alzheimer’s disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene. Nature. 1991;353:844–846. doi: 10.1038/353844a0. [DOI] [PubMed] [Google Scholar]
- 21.Cruts M., Hendriks L., Van Broeckhoven C. The presenilin genes: A new gene family involved in alzheimer disease pathology. Hum. Mol. Genet. 1996;5:1449–1455. doi: 10.1093/hmg/5.Supplement_1.1449. [DOI] [PubMed] [Google Scholar]
- 22.Sugarman M.C., Yamasaki T.R., Oddo S., Echegoyen J.C., Murphy M.P., Golde T.E., Jannatipour M., Leissring M.A., LaFerla F.M. Inclusion body myositis-like phenotype induced by transgenic overexpression of beta app in skeletal muscle. Proc. Natl. Acad. Sci. USA. 2002;99:6334–6339. doi: 10.1073/pnas.082545599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris G.P., Clark I.A., Vissel B. Inconsistencies and controversies surrounding the amyloid hypothesis of alzheimer’s disease. Acta Neuropathol. Commun. 2014;2:135. doi: 10.1186/s40478-014-0135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joshi P., Turola E., Ruiz A., Bergami A., Libera D.D., Benussi L., Giussani P., Magnani G., Comi G., Legname G., et al. Microglia convert aggregated amyloid-beta into neurotoxic forms through the shedding of microvesicles. Cell Death Differ. 2014;21:582–593. doi: 10.1038/cdd.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bu X.L., Xiang Y., Jin W.S., Wang J., Shen L.L., Huang Z.L., Zhang K., Liu Y.H., Zeng F., Liu J.H., et al. Blood-derived amyloid-β protein induces alzheimer’s disease pathologies. Mol. Psychiatry. 2017 doi: 10.1038/mp.2017.204. [DOI] [PubMed] [Google Scholar]
- 26.Wang S., Mims P.N., Roman R.J., Fan F. Is beta-amyloid accumulation a cause or consequence of alzheimer’s disease? J. Alzheimer’s Parkinsonism Dement. 2016;1:007. [PMC free article] [PubMed] [Google Scholar]
- 27.Friedrich R.P., Tepper K., Rönicke R., Soom M., Westermann M., Reymann K., Kaether C., Fändrich M. Mechanism of amyloid plaque formation suggests an intracellular basis of aβ pathogenicity. Proc. Natl. Acad. Sci. USA. 2010;107:1942–1947. doi: 10.1073/pnas.0904532106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gouras G.K., Tampellini D., Takahashi R.H., Capetillo-Zarate E. Intraneuronal β-amyloid accumulation and synapse pathology in alzheimer’s disease. Acta Neuropathol. 2010;119:523–541. doi: 10.1007/s00401-010-0679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y.-W., Thompson R., Zhang H., Xu H. App processing in alzheimer’s disease. Mol. Brain. 2011;4:3. doi: 10.1186/1756-6606-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duffy J.B. Gal4 system in drosophila: A fly geneticist’s swiss army knife. Genesis. 2002;34:1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- 31.Brenner S. The genetics of caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawashima T., Zwart M.F., Yang C.T., Mensh B.D., Ahrens M.B. The serotonergic system tracks the outcomes of actions to mediate short-term motor learning. Cell. 2016;167:933–946. doi: 10.1016/j.cell.2016.09.055. [DOI] [PubMed] [Google Scholar]
- 33.Edward D., Levin D.T.C. Methods of Behavior Analysis in Neuroscience. 2 ed. CRC Press/Taylor & Francis; Boca Raton, FL, USA: 2009. [Google Scholar]
- 34.Fenno L., Yizhar O., Deisseroth K. The development and application of optogenetics. Annu. Rev. Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guglielmi G., Barry J.D., Huber W., De Renzis S. An optogenetic method to modulate cell contractility during tissue morphogenesis. Dev. Cell. 2015;35:646–660. doi: 10.1016/j.devcel.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Idevall-Hagren O., Dickson E.J., Hille B., Toomre D.K., De Camilli P. Optogenetic control of phosphoinositide metabolism. Proc. Natl. Acad. Sci. USA. 2012;109:E2316–E2323. doi: 10.1073/pnas.1211305109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kennedy M.J., Hughes R.M., Peteya L.A., Schwartz J.W., Ehlers M.D., Tucker C.L. Rapid blue-light-mediated induction of protein interactions in living cells. Nat. Methods. 2010;7:973–975. doi: 10.1038/nmeth.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]