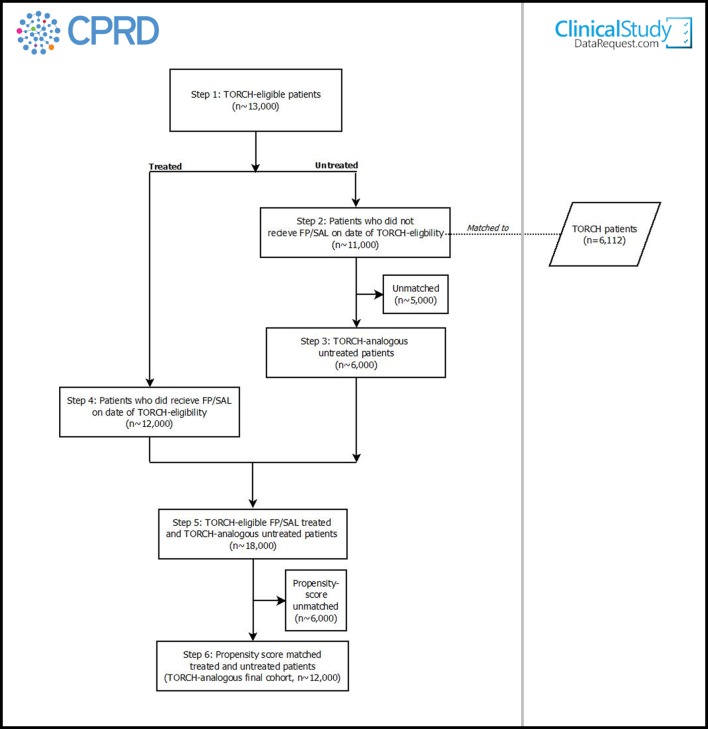
Figure 2.
Flow chart illustrating the planned selection of CPRD participants for objective 1 of the COPD real-world medicines effects study. Note in relation to step 5 (n~18 000) compared with step 1 (n~13 000): approximately 90% of the treated patients will also have been eligible as untreated patients, as they did not receive FP/SAL on their TORCH-eligibility date. This means that they will have at least one period of time during which they are untreated-eligible but then did subsequently go on to receive FP/SAL (meaning they have at least one period of time during which they are treated-eligible). If a person is included as both a treated and untreated participant, they will be contributing different periods of their person-time to each cohort (pre-FP/SAL treatment for the untreated vs post-FP/SAL treatment for treated), and this is handled in the analysis by assigning different index dates. COPD, chronic obstructive pulmonary disease; CPRD, UK Clinical Practice Research Datalink; FP/SAL, fluticasone propionate+salmeterol.