Abstract
Purpose
The Nineteen and Up study (19Up) assessed a range of mental health and behavioural problems and associated risk factors in a genetically informative Australian cohort of young adult twins and their non-twin siblings. As such, 19Up enables detailed investigation of genetic and environmental pathways to mental illness and substance misuse within the Brisbane Longitudinal Twin Sample (BLTS).
Participants
Twins and their non-twin siblings from Queensland, Australia; mostly from European ancestry. Data were collected between 2009 and 2016 on 2773 participants (age range 18–38, 57.8% female, 372 complete monozygotic pairs, 493 dizygotic pairs, 640 non-twin siblings, 403 singleton twins).
Findings to date
A structured clinical assessment (Composite International Diagnostic Interview) was used to collect lifetime prevalence of diagnostic statistical manual (4th edition) (DSM-IV) diagnoses of major depressive disorder, (hypo)mania, social anxiety, cannabis use disorder, alcohol use disorder, panic disorder and psychotic symptoms. Here, we further describe the comorbidities and ages of onset for these mental disorders. Notably, two-thirds of the sample reported one or more lifetime mental disorder.
In addition, the 19Up study assessed general health, drug use, work activity, education level, personality, migraine/headaches, suicidal thoughts, attention deficit hyperactivity disorder (ADHD) symptomatology, sleep–wake patterns, romantic preferences, friendships, familial environment, stress, anorexia and bulimia as well as baldness, acne, asthma, endometriosis, joint flexibility and internet use.
The overlap with previous waves of the BLTS means that 84% of the 19Up participants are genotyped, 36% imaged using multimodal MRI and most have been assessed for psychological symptoms at up to four time points. Furthermore, IQ is available for 57%, parental report of ADHD symptomatology for 100% and electroencephalography for 30%.
Future plans
The 19Up study complements a phenotypically rich, longitudinal collection of environmental and psychological risk factors. Future publications will explore hypotheses related to disease onset and development across the waves of the cohort. A follow-up study at 25+years is ongoing.
Keywords: twins, comorbidity, prevalence, substance misuse
Strengths and limitations of this study.
Large sample size (n=2773; 369 monozygotic and 494 dizygotic twin pairs): provides statistical power (>0.8) to detect heritability >0.25, shared environment influences >0.2 and a genetic correlation >0.3 (when heritability for both phenotypes >20%).
Well-characterised lifetime psychiatric diagnoses and substance use (DSM-IV abuse and dependence criteria, for a wide variety of licit and illicit substances, including non-medical use of over-the-counter and prescription substances).
Rich biological samples: hair sample (cortisol) and longitudinal blood samples (vitamin D, antibodies, metabolites, gene expression, genome-wide association study (GWAS)).
Longitudinal design: most participants have been assessed at 12, 14, 16 and 21 years. Repeated observations within 19Up, to study scores and diagnoses stability and reliability.
Multimodal imaging: 36% of participants underwent structural and functional MRI and diffusion tensor imaging (DTI).
Introduction
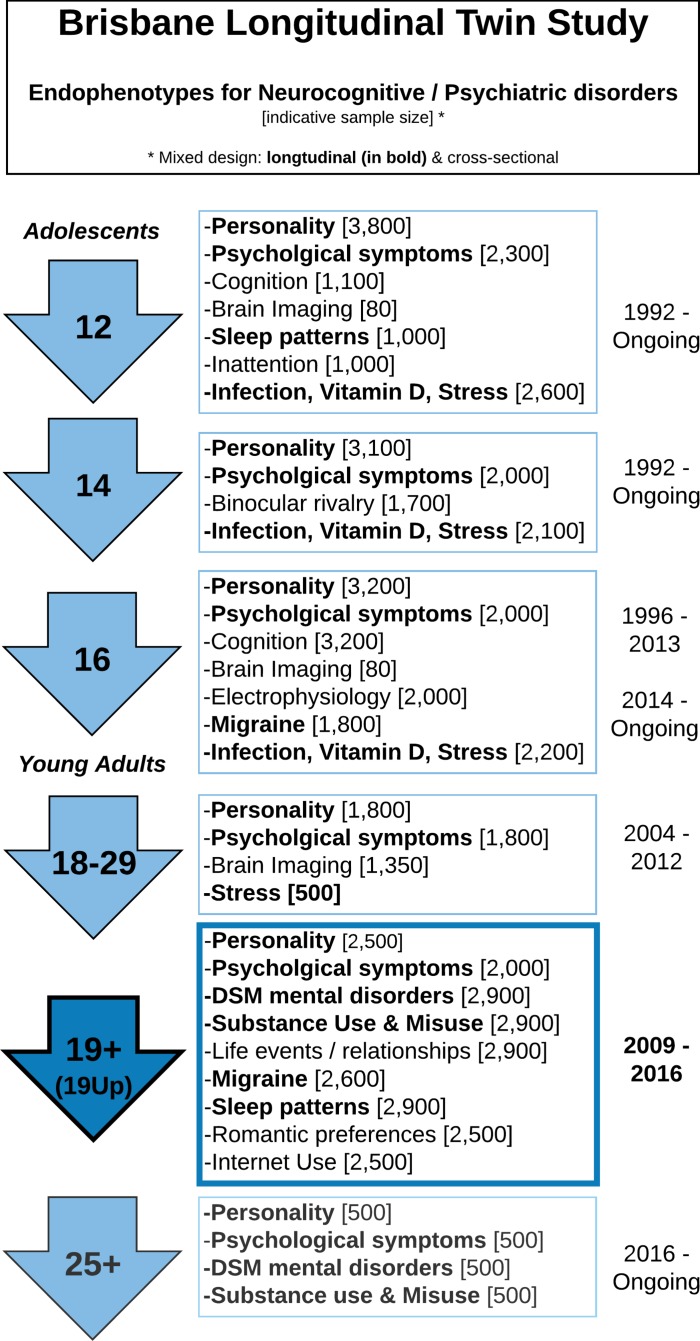
Between 2009 and 2016, the Nineteen Up Study (19Up: Mapping neurobiological changes across mental health stages, see1 for study protocol) assessed a range of mental health and behavioural problems and associated risk factors in a genetically informative Australian population sample of young adult twins and their non-twin siblings. These individuals are part of the ongoing Brisbane Longitudinal Twin Study (BLTS, or Brisbane Adolescent Twin Study: BATS,1 2 which began in 1992 when twins were recruited from primary and secondary schools in the greater Brisbane area via media appeals and by word of mouth. A key strength of 19Up is the ability to link twin data with a phenotypically rich, longitudinal collection of environmental and psychological risk factors including personality, psychiatric phenotypes and diagnostic outcomes, neurobiological correlates such as brain imaging and genome-wide association data (figure 1). As such, 19Up enables detailed investigation of the genetic and environmental pathways to mental illness as well as substance use and misuse.
Figure 1.
Summary of the Brisbane Longitudinal Twin Sample data collection. Longitudinal: vitamin D; infections (antibodies); neuroticism junior Eysenck personality questionnaire (JEPQ) neuroticism-extraversion-openness inventory (NEO); psychiatric signs (SPHERE). Cross-sectional: hair cortisol; cognition (verbal, performance IQ, working memory, information processing); binocular rivalry (rivalry rate); brain imaging (multimodal MRI); substance use (alcohol, tobacco, recreational drugs); sleep patterns (actigraphy); psychiatric diagnoses (Composite International Diagnostic Interview); life events/social support/relationships (eg, early home environment, family relationships, traumatic events, socioeconomic factors). *Sample size in only indicative as many of the early waves are still recruiting new participants. Phenotypes in bold are collected longitudinally, other are cross-sectional.
The 19Up study complements and extends earlier BLTS and BATS studies conducted during adolescence (figure 1)3–12 by providing a detailed assessment of mental health and substance use and misuse at a young adult age. The study was organised around collecting lifetime diagnoses of substance misuse and common mood disorders, but also a wide range of behavioural and subclinical assessments, as well as updates on phenotypes previously collected in the BLTS (figure 1). Finally, the 19Up data collection was also designed to contribute to twin and genetic consortia in psychiatry, personality and brain imaging. We hope that the description of the full sample below will assist future publications and encourage further collaborations making use of the rich 19Up data (see ‘Collaboration’ section).
Cohort description
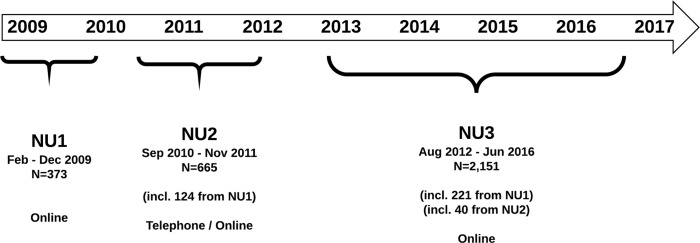
Data were collected in three waves (NU1, NU2, NU3) between February 2009 and June 2016 (figure 2). Initially, mental health scores were collected via an online survey (NU1, n=373), which was replaced in September 2010 by a more detailed online questionnaire complemented by a Computer-Assisted Telephone Interview (CATI) of the Composite International Diagnostic Interview (CIDI13) (NU2, n=665). Beginning 1 July 2012, the online survey and CATI instruments were then merged into a unique, more economical online protocol, divided into three sections (see table 1 and figure 2). Ascertainment began with the oldest BLTS adult twins and their non-twin siblings in order to obtain data from individuals who had passed through the peak age range for the onset for substance use disorders, anxiety and mood disorders.14–17
Figure 2.
Timeline of the Nineteen and Up study data collection. Because of the changes in protocols, participants from NU1 were all reapproached to complete the following NU2 or NU3 waves. The vast majority (92%) then completed NU2 or NU3. Despite an interval of several years, these data provide an opportunity to examine test–retest reliability of scores or compare collection methods (eg, self-report online vs telephone interview). In addition to providing insight into the validity of the measures, these data are important for twin modelling as the stability of a phenotype or diagnosis sets an upper limit for the heritability.
Table 1.
Demographics of the final sample and detail by wave
| Total* | NU1 | NU2 | NU3 | |
| N invited | 4156† | 841† | 2240† | 3374† |
| % females (95% CI) | 50.5% (48.9 to 52.1) | 54.8% (51.4 to 58.2) | 50.4% (48.3 to 52.4) | 49.7% (48.0 to 51.4) |
| N completed (response rate) | 2773 (67%) | 373 (44%) | 665 (30%) | 2151 (64%) |
| Mean age (SD) (range) | 26.1 (4.1) (18.7–38.6) | 24.7 (3.3) (18.4–30.4) | 27.4 (2.9) (20.6–38.6) | 25.7 (4.3) (18.7–38.3) |
| % females (95% CI) | 57.8% (55.9 to 59.6) | 62.9% (57.7 to 67.8) | 58.2% (54.3 to 62.0) | 57.6% (55.4 to 60.0) |
| Marital status % (n) | NA‡ | |||
| Married | 21.6% (599) | 28.1% (187) | 19.4% (418) | |
| Separated | 1.0% (28) | 0.9% (6) | 1.1% (23) | |
| Divorced | 1.0% (28) | 1.4% (9) | 0.9% (20) | |
| Widowed | 0.1% (3) | 0.1% (3) | ||
| Never married | 76.3% (2115) | 69.6% (463) | 78.4% (1687) | |
| Have children % (95% CI) | 18.3% (16.9 to 19.8) | NA‡ | 22.1% (19.0 to 25.5) | 17.3% (15.7 to 19.0) |
| Highest education level§ % (n) | NA‡ | |||
| No formal education | 0.0% (1) | 0.0% (0) | 0.05% (1) | |
| Primary school | 0.0% (0) | 0.0% (0) | 0.0% (0) | |
| Junior secondary school | 1.8% (51) | 1.5% (10) | 2.1% (45) | |
| Senior secondary school | 16.2% (449) | 14.4% (96) | 16.9% (364) | |
| Certificate or diploma | 24.3% (675) | 29.3% (195) | 23.2% (498) | |
| Degree | 44.7% (1239) | 39.5% (263) | 45.7% (983) | |
| Postgraduate diploma, masters, PhD | 12.8% (354) | 15.2% (101) | 11.9% (256) | |
| Don’t know/prefer not to answer | 0.1% (4) | 0.0% (0) | 0.18% (4) |
*NU1 data is in part reported in ref.,1 but not included in the total sample or used in this analysis as most of the participants (345 out of 373: 92%) later completed NU2 or NU3.
†4156 unique individuals were invited to participate in the 19Up, but some were invited in several waves. Participants invited in NU1 were all reinvited in NU2. They were also invited as part of NU3 if they had not completed NU2 and not refused to be recontacted. Forty participants of NU2 also completed NU3.
‡Succinct demographics for NU1 were collected as part of a different study on political views and economical games and different questions were used.
§Participants were asked about their highest level of education (completed or partially completed) at the time of questionnaire.
19Up, Nineteen and Up study.
The NU1 questionnaire assessed general health, mental health symptomatology (Somatic and Psychological Health Report: SPHERE-12,18–21 KESSLER-622), use of alcohol, nicotine,23 cannabis and other substances including the non-medical use of prescriptions substances; migraine and headaches, inattention (The Strengths and Weaknesses of ADHD symptoms and Normal behaviour rating scale: SWAN)24 and baldness (online supplementary file 2). The following waves (NU2 and 3) also included structured clinical assessment (CIDI),13 self-reported symptoms of mania (Altman Self-Rating Mania Scale25), suicidal thoughts, sleep quality and sleep–wake patterns (Pittsburgh Sleep Quality Index26 and Insomnia Severity Index27) and general demographics, where participants were asked about their work activity/occupation, level of education, quality of friendships, familial environment (Parental Bonding Instrument28) and exposure to adversity (List of Threatening Experiences29–31). Finally, sections of the NU2 and 3 questionnaires also assessed personality,32–34 acne, asthma, anorexia, bulimia, endometriosis, joint flexibility, romantic preferences and internet use (online supplementary file 2, figure 1, see also ref.1).
bmjopen-2017-018959supp002.pdf (30.9KB, pdf)
We used the CIDI13 to identify lifetime DSM-IV diagnoses of major depressive disorder (MDD), mania, social anxiety, cannabis dependence, alcohol dependence and panic disorder (with and without agoraphobia), basic epidemiology of ecstasy and methamphetamine use, as well as psychotic symptoms. These narrow DSM diagnoses can be used for collaborations with consortia and have served to extract an MDD case–control sample for the ENIGMA-MDD consortium,35 36 with a tight control of the comorbidities present in the sample.
In addition, we derived alternative diagnoses of depressive, manic and hypomanic episodes that focus on the core diagnostic criteria (criteria A and B of the DSM) and do not enforce the DSM-IV exclusions related to substance use, putative cause of the disorder (eg, bereavement in depression) or hierarchy of DSM disorders (see online supplementary file 1). These represent broader definitions of the disorders, with greater rates of (co)morbidity and will be preferred for future studies of psychiatric trajectories in the BLTS (see online supplementary file 1 for more details about the clinical syndrome definitions).
bmjopen-2017-018959supp001.pdf (48.3KB, pdf)
Of the 4156 individuals invited to participate in the study, 67% of the twins and non-twin siblings provided complete data. Overall, females were slightly over-represented among the 19Up respondents: comprising 50.5% (95% CI 48.9 to 52.1) of the invited population but 57.8% (55.9 to 59.6) of the actual ascertained participants (table 1). Across the last two waves (NU2 and 3), 2773 twins and non-twin siblings completed the demographic and CIDI questionnaires (mean age=26.1, SD=4.1, range 18–38, 57.8% female, 369 complete monozygotic pairs, 494 dizygotic pairs). Due to the ascertainment strategy employed, participants who completed the telephone interview (NU2) were significantly older than participants who completed the online survey (mean age 27.4 (range=20.6–38.6) vs 25.7 (range=18.7–38.3), t=11.6, P=2.2E-16) but the sex ratio was comparable across the two waves: 58.2% vs 57.6%, χ2=0.035, P=0.85 (table 1). The mean age of participants in NU1 was 24.7 (range=18.4–30.4) with a sex ratio of 62.9%. Ethnically, the cohort reflects the population structure of families with twins in Queensland at the time of recruitment, with a majority of participants of European ancestry and minorities of predominantly Asian ancestry.1
Non-twin participants were on average older than their twin siblings (26.9 vs 25.8, t=5.70, P=1.5e-8) and were more likely to be married (26.2% vs 20.0%, χ2=14.5, P=0.0057) with children (22.3% vs 17.1%, χ2=8.66, P=0.0033), but twins and non-twins siblings did not differ in education level (χ2=2.2, df=7, P=0.94) or sex ratio (χ2=0.017, P=0.89).
All participants had been invited to complete previous BLTS1 2 studies (figure 1). Height, weight, personality, psychiatric signs, sleep patterns, migraine and blood samples (haematological and immunological measures: eg, antibodies markers of infections, vitamin D) were collected longitudinally in the BLTS, with up to five time points for some phenotypes (figure 1). In addition, genome-wide single nucleotide polymorphism (SNP) genotypes are currently available for 84% (n=2324) of the 19Up participants. These data have been imputed and quality controlled (see ref.1 for details) using state-of-the-art procedures,37–40 which allow combining data from different arrays, and currently represent a more cost-effective approach to study complex human traits than whole-genome sequencing (at current prices).41
Multimodal brain MRI was collected cross-sectionally and is available for 987 (36%) of the 19Up respondents (see ref.42 for all details). Further assessments during adolescence are available for part of 19Up: cognition (available for 56.8% of the sample), parental report of ADHD symptomatology (100%), binocular rivalry (19.6%) and electroencephalography (30.4%) (see refs.2 4 7 43 for details about these waves). Finally, a follow-up study of all 19Up participants has been funded and is currently ongoing (expected mean age at follow-up 25).
Findings to date
Among the full DSM-IV diagnostic criteria, social anxiety and MDD were the most prevalent diagnoses (both at 17.5%, n=486), followed by panic disorder without agoraphobia (1.5%, n=42) and panic disorder with agoraphobia (0.9%, n=24). In addition, 14.3% (n=397) of the respondents reported a past panic attack and 0.5% (n=14) qualified for a manic episode (table 2). Lifetime prevalence of mental disorders were slightly increased when the lower threshold of cases meeting DSM-IV clinical criteria (A and B) was applied: 25.8% (n=715) for MDD, 6.3% (n=175) for hypomania and 2.0% (n=56) for mania.
Table 2.
Prevalence of DSM-IV diagnoses in the 19Up study
| Total prevalence % (95% CI) |
Prevalence males % (95% CI) |
Prevalence females % (95% CI) |
P values males versus females | Prevalence NU2 % (95% CI) |
Prevalence NU3 % (95% CI) |
P values NU2 versus NU3 | |
| Affective disorders | |||||||
| MDD | 17.5% (16.1 to 19.0) |
13.8% (11.9 to 15.9) |
20.3% (18.3 to 22.3) |
1.06E-05 | 15.2% (12.6 to 18.2) |
18.3% (16.6 to 20.0) |
0.078 |
| Social anxiety | 17.5% (16.1 to 19.0) |
13.2% (11.3 to 15.3) |
20.7% (18.8 to 22.8) |
3.2E-07 | 16.2% (13.6 to 19.3) |
17.9% (16.3 to 19.7) |
0.35 |
| Panic disorder (with agoraphobia) | 0.9% (0.57 to 1.3) |
0.3% (0.1 to 0.8) |
1.3% (0.8 to 2.0) |
5.9E-3*† | 1.1% (0.5 to 2.3) |
0.8% (0.5 to 1.3) |
0.72 |
| Panic disorder (without agoraphobia) | 1.5% (1.1 to 2.1) |
0.9% (0.4 to 1.6) |
2.0% (1.4 to 2.8) |
0.023† | 1.7% (0.9 to 3.0) |
1.5% (1.0 to 2.1) |
0.88 |
| Panic attack | 14.3% (13.0 to 15.7) |
9.2% (7.6 to 11.0) |
18.1% (16.3 to 20.1) |
4.5E-11 | 15.6% (13.0 to 18.7) |
13.9% (12.5 to 15.5) |
0.29 |
| Manic episode | 0.5% (0.3 to 0.9) |
0.7% (0.3 to 1.4) |
0.4% (0.2 to 0.9) |
0.39 | 0.6% (0.2 to 1.6) |
0.5% (0.2 to 0.9) |
0.93* |
| Substance use | |||||||
| Lifetime use of any drug‡ | 57.8% (56.0 to 59.7) |
63.2% (60.4 to 66.0) |
53.9% (51.4 to 56.4) |
1.0E-06 | 62.3% (58.4 to 65.9) |
56.5% (54.3 to 58.6) |
9.4E-3† |
| Cannabis abuse | 11.6% (10.5 to 12.9 |
17.0% (14.9 to 19.3) |
7.7% (6.44 to 9.11) |
5.6E-14 | 15.3% (12.7 to 18.4) |
10.4% (9.2 to 11.8) |
7.5E-4 |
| Cannabis dependence | 6.8% (5.9 to 7.8) |
9.8% (8.2 to 11.7) |
4.6% (3.7 to 5.8) |
1.1E-07 | 10.7% (8.5 to 13.3) |
5.6% (4.7 to 6.7) |
8.9E-06 |
| Alcohol abuse | 33.8% (32.1 to 35.6) |
40.2% (37.4 to 43.1) |
29.2% (27.0 to 31.5) |
2.1E-09 | 36.7% (33.0 to 40.5) |
32.9% (30.9 to 35) |
0.081 |
| Alcohol dependence | 28.0% (26.3 to 29.7) |
35.4% (32.7 to 38.2) |
22.6% (20.6 to 24.7) |
1.66E-13 | 32.8% (29.2 to 36.5) |
26.5% (24.6 to 28.4) |
0.0019 |
| Core diagnostic criteria | |||||||
| Depression | 25.8% (24.2 to 27.5) |
20.3% (18.1 to 22.8) |
29.8% (27.6 to 32.1) |
2.7E-08 | 25.1% (21.9 to 28.6) |
26% (24.2 to 27.9) |
0.68 |
| Hypomania | 6.3% (5.5 to 7.3) |
6.3% (5.0 to 7.8) |
6.4% (5.3 to 7.7) |
0.97 | 5.7% (4.1 to 7.8) |
6.5% (5.5 to 7.7) |
0.52 |
| Mania | 2.0% (1.5 to 2.6) |
2.1% (1.4 to 3.1) |
2.0% (1.4 to 2.8) |
1 | 1.1% (0.5 to 2.3) |
2.3% (1.7 to 3.1) |
0.06 |
| N | 2773 | 1170 | 1603 | 665 | 2151 | ||
Significant P values after multiple testing correction are highlighted in bold. Analyses performed using a χ2 test (1 degree of freedom) unless stated. Cells report prevalence % (95% CI).
*Fisher’s exact test used.
†Would not survive multiple testing correction of 0.05/22=0.0022.
‡Illicit drug or non-medical use of prescription drug. Participants are asked specifically about cocaine, amphetamine-type stimulants, inhalants, sedatives or sleeping pills, hallucinogens, opioids, party drugs (ecstasy, ketamine, GHB), over-the-counter/prescription pain killers and analgesics for non-medical purposes, over-the-counter/prescription stimulants for non-medical purposes, or other.
19Up, Nineteen and Up study; MDD, major depressive disorder.
Over half (57.8%, n=1604) of the samples reported illicit substance use including the non-medical use of over-the-counter and prescription substances (table 2). Among the substance use disorders, DSM-IV diagnoses of lifetime alcohol abuse (Alc-ab) and dependence (Alc-dep) were the most prevalent at 33.8% (n=938) and 28.0% (n=776), respectively. In comparison, lifetime prevalence of cannabis abuse (can-ab) and dependence (can-dep) were 11.6% (n=322) and 6.8% (n=189, table 2).
Among participants, 7.1% (n=196) had experienced one or more psychotic symptoms in their lifetime (table 3). Visual hallucinations were the most common, occurring in 3.9% (n=107) of the sample, followed by auditory hallucinations (3.3%, n=91), while delusions were rare (thoughts insertion and thought broadcasting: 0.4% (n=11) ‘made’ feelings and impulses: 0.3% (n=9), delusions of references: 1.1% (n=29) and delusions of persecution: 0.9% (n=25)).
Table 3.
Prevalence of psychotic symptoms
| Total prevalence | Prevalence males | Prevalence females | P values males versus females | Prevalence NU2 | Prevalence NU3 | P values NU2 versus NU3 | |
| Psychotic symptoms in the last 12 months | 2.7% (2.2 to 3.4) | 2.9% (2.1 to 4.1) | 2.6% (1.9 to 3.5) | 0.66 | 2.4% (1.4 to 4.0) | 2.8% (2.2 to 3.6) | 0.68 |
| Lifetime presence of any psychotic symptoms* | 7.1% (6.2 to 8.1) | 7.4% (6.0 to 9.1) | 6.8% (5.6 to 8.2) | 0.57 | 7.8% (6.0 to 10.2) | 6.8% (5.8 to 8.0) | 0.44 |
| Visual hallucinations | 3.9% (3.2 to 4.7) | 3.6% (2.6 to 4.9) | 4.1% (3.2 to 5.2) | 0.60 | 4.2% (2.8 to 6.1) | 3.8% (3.0 to 4.7) | 0.73 |
| Auditory hallucinations | 3.3% (2.7 to 4.0) | 3.9% (2.9 to 5.3) | 2.8% (2.1 to 3.8) | 0.13 | 3.3% (2.1 to 5.1) | 3.3% (2.6 to 4.2) | 1.0 |
| Delusions: thought insertion and thought broadcasting | 0.4% (0.21 to 0.73) | 0.6% (0.26 to 1.3) | 0.3% (0.1 to 0.6) | 0.22† | 0.6% (0.2 to 1.7) | 0.3% (0.2 to 0.7) | 0.30† |
| Delusions: ‘made’ feelings and impulses | 0.3% (0.2 to 0.6) | 0.3% (0.1 to 0.9) | 0.3% (0.1 to 0.7) | 1† | 0.6% (0.2 to 1.6) | 0.2% (0.1 to 0.6) | 0.23† |
| Delusions of reference | 1.1% (0.7 to 1.5) | 1.5% (0.9 to 2.4) | 0.7% (0.4 to 1.3) | 0.11 | 2.0% (1.1 to 3.4) | 0.7% (0.5 to 1.3) | 0.015‡ |
| Delusions of persecution | 0.9% (0.6 to 1.3) | 0.9% (0.5 to 1.7) | 0.9% (0.5 to 1.6) | 0.98 | 1.1% (0.5 to 2.3) | 0.9% (0.5 to 1.4) | 0.81 |
| N | 2773 | 1170 | 1603 | 665 | 2151 |
Cells report prevalence % (95% CI). P values calculated using a χ2 test unless stated otherwise.
*Includes any of the psychotic symptoms.
†Fisher’s exact test used.
‡Would not survive multiple testing correction of 0.05/16=0.0031.
The prevalence for (DSM-IV) mood disorders was higher in females than males (table 2). For example, females were almost 1.5-fold as likely than males to meet criteria for MDD (DSM-IV narrow diagnosis: 20.3% F, 13.8% M, χ2=14.2, P=1.6E-4; broad criteria: 29.8% F, 20.3% M, χ2=30.9, P=2.7E-8) and social anxiety (20.7% F, 13.2% M, χ2=26.4, P=3.2E-7). Panic disorder was at least three times more prevalent in females than males (with agoraphobia 1.3% F, 0.3% M, χ2=7.6, P=5.9E-3, without agoraphobia 2.0% F, 0.1% M, χ2=5.2, P=0.02). Similarly, panic attack(s) were more common in females than males (18.1% F 9.2% M, χ2=43.4, P=4.5E-11). No significant sex differences were observed in the prevalence of manic episode or psychotic symptoms (table 2).
Substance use disorders were more common in males (can-ab: 17.0% M, 7.7% F, χ2=56.5, P=5.6E-14; can-dep: 9.8% M, 4.6% F, χ2=28.1, P=1.1E-7; Alc-ab: 40.2% M, 29.2% F, χ2=35.9, P=2.1E-9; Alc-dep: 35.4% M, 22.6% F, χ2=54.3, P=1.7E-13). Use of any illegal drug (including the non-medical use of over-the-counter and prescription substances) was also significantly higher in males compared with females (63.2% M, 53.9% F, χ2=23.8, P=1.0E-6, table 2). For Alc-dep the prevalence was higher in the CATI (NU2) compared with online (NU3) participants (table 2, online supplementary file 1), which could be partly explained by the older age of the NU2 sample compared with the NU3 participants (table 1).
Ages of onset was comparable across sexes for all DSM-IV diagnoses, but it varied substantially across disorders (ie, 11.5 years for social anxiety, 18.5 years for panic disorder and around 20 years for MDD and can-dep; table 4). Age of onset was not available for alcohol dependence as only the age at initiation (16.0 years) was collected. The mean ages of onset for manic episode, panic attack and psychotic symptoms were 19.6, 17.6 and 15.7 years, respectively.
Table 4.
Differences in the age of onset for males and females
| Mean age of onset (SD) | Mean age of onset males (SD) | Mean age of onset females (SD) | P values males versus females | |
| Manic episode | 19.6 (5.0) | 19.2 (5.4) | 20.0 (5.6) | 0.87 |
| First psychotic symptom | 15.7 (6.4) | 15.0 (6.4) | 16.2 (6.3) | 0.21 |
| Major depressive disorder | 20.6 (5.1) | 21.3 (5.4) | 20.3 (5.0) | 0.038 |
| Social anxiety | 11.6 (5.0) | 11.3 (4.7) | 11.7 (5.1) | 0.42 |
| Cannabis abuse | 19.7 (3.1) | 19.8 (3.0) | 19.6 (3.2) | 0.65 |
| Cannabis dependence | 19.8 (3.0) | 20. 0 (2.9) | 19.6 (3.0) | 0.39 |
| Cannabis initiation | 17.7 (4.1) | 17.5 (2.8) | 17.9 (4.9) | 0.05 |
| Alcohol initiation | 16.0 (1.8) | 15.8 (1.8) | 16.1 (1.8) | 1.20E-05 |
| Panic attack | 17.6 (5.5) | 16.8 (5.9) | 17.9 (5.3) | 0.11 |
| Panic disorder with or without agoraphobia | 18.7 (5.7) | 20.4 (5.0) | 18.3 (5.9) | 0.22 |
Age of onset was not collected for alcohol abuse, dependence and use disorder. We reported age at first drink (initiation), age at first intoxication and age of ‘regular use’ (one drink a month for 6 months) have also been collected.
After multiple testing correction are shown in bold.
The lifetime prevalence reported above highlights how common mental disorders may be in an unselect sample of young Australian adult twins. Together with the large sample size of the present study and the longitudinal detailed phenotyping, the 19Up study is suited to identifying early markers of mental health risk and shed light on the pathways to psychiatric disorders.
Discussion
Prevalence of MDD (DSM-IV) in 19Up was higher than that reported by the 2007 National Survey of Mental Health and Wellbeing (7%)44 the WHO World Mental Health Surveys (12.8%)45 or in a large adolescent cohort from the USA46 while comparable prevalence have been reported in New Zealand47 48 and in Australian women.49 These results highlight that if overall MDD is a common condition in high-income countries47 its observed prevalence depends on the age of the respondent. Similarly, the younger age of participants in the 19Up study likely explains the younger mean age of onset for MDD compared with published research.50 51 Our lifetime prevalence for social anxiety was higher than that previously reported in Australia,49 52 New Zealand48 and the USA,53 but similar to the prevalence in a separate sample of Australian twins.54 Age of onset for social anxiety in the 19Up study was comparable to previous reports.48 50 51
The prevalence of panic disorder without agoraphobia has been reported to be between 2.3% and 10.9%,46 48 53 55 higher than our findings (1.5%). Published prevalences of panic disorder with agoraphobia are also higher than in our study (1.1%–3.8%53 55 vs. 0.9%), which could be due to our limited age range.
Population studies found between 5.4% and 6.2% of Australians meet criteria for cannabis abuse.56 57 However, the prevalence has been reported higher (11.4%) within 25–44 years,57 comparable to our results for can-ab/can-dep (11.6%/6.8%). In addition, we found 28.0% of those in 19Up were alcohol dependent (33.8% with alcohol abuse), consistent with previous publications,53 58 despite differences in recruitment and age of the respondents. The differences with studies that reported lower prevalence of alcohol dependence in both Australia (3.8%)57 and New Zealand (4%)48 could be due to differences in data collection (face-to-face vs online), recruitment and demographics. Finally, prevalence of use of any illegal substance (and misuse of prescription drug) was comparable to previously available Australian data (57.8% compared with 51.2% in 20–29 years, 59.3% in 30–39).48 59
Prevalence of psychotic symptoms in the 19Up study (7.1%) matched results of the largest studies to date,60 61 while other studies reported prevalence between 5.5%62 and 11.7%.63 Hallucinations were the most common symptom, as previously reported,60 and the prevalence of specific symptoms was comparable to our results.60 62 63 However, we found a younger mean age of onset for psychotic symptoms (15.7 years) than previous studies,64 which could be attributed to the age range of the cohort.
Overall, the sex differences matched previous studies. Affective disorders were all more prevalent in women46 48 54 65–67 while substance use disorders (and abuse or dependence) were more common in men.16 46 48 59
In our sample, comorbidities were widespread across the diagnoses (table 5, online supplementary file 3) consistent with previous epidemiological results45 50 52 57 58 68–70 and explained in part by genetic correlations between psychiatric diagnoses.71 72 About 60.4% of our sample met the criteria for at least one DSM-IV diagnosis in their lifetime, with 18.0% of these reporting a second lifetime diagnoses, 9.1% reporting three diagnoses and 6.8% reporting four or more lifetime diagnoses. When considering only the core symptoms of depression, core mania/hypomania and previous psychotic experiences, 31.9% of the sample reported at least one clinical syndrome (24.0% with exactly one clinical syndrome, 6.7% with two lifetime clinical syndrome and 1.2% with the three clinical syndromes).
Table 5.
Proportion of people with one DSM-IV disorder (rows) who also have another disorder (columns)
| MDD | Social anxiety | Cannabis abuse | Cannabis dependence | Alcohol abuse | Alcohol dependence | Panic with agora | Panic without agora | Panic attack | Manic episode | Psychotic symptoms | Any illegal substance use* | ||
| MDD | Cases | – |
33.7%
(29.6 38.2) |
9.5% (7.1 to 12.5) |
5.8% (3.9 to 8.3) | 34.8% (30.6 to 39.2) |
30% (26 to 34.4) |
1.9% (0.9 to 3.6) |
4.3%
(2.8 to 6.6) |
31.1%
(27 to 35.4) |
1% (0.4 to 2.5) |
11.3%
(8.7 to 14.6) |
60.1% (55.6 to 64.4) |
| Controls |
14.1%
(12.7 to 15.6) |
12.1% (10.8 to 13.5) |
7% (6 to 8.2) |
33.6% (31.7 to 35.6) |
27.5% (25.7 to 29.4) |
0.7% (0.4 to 1.1) |
0.9%
(0.6 to 1.4) |
10.8%
(9.5 to 12.1) |
0.4% (0.2 to 0.8) |
6.2%
(5.2 to 7.2) |
57.4% (55.3 to 59.4) |
||
| P values | 7.80E-25 | 0.12 | 0.36 | 0.66 | 0.29 | 0.021 | 7.70E-08 | 8.30E-31 | 0.15 | 8.60E-05 | 0.29 | ||
| Social anxiety | Cases |
33.7%
(29.6 to 38.2) |
– | 15.2% (12.2 to 18.8) |
9.3% (6.9 to 12.3) |
36.8% (32.6 to 41.3) |
34.2%
(30.0 to 38.6) |
2.1% (1.0 to 3.9) |
4.1%
(2.6 to 6.4) |
30.5%
(26.4 to 34.8) |
1.6%
(0.8 to 3.3) |
9.7% (7.3 to 12.7) |
62.1% (57.6 to 66.4) |
| Controls |
14.1%
(12.7 to 15.6) |
10.8% (9.6 12.2) |
6.3% (5.4 to 7.4) |
33.2% (31.3 to 35.2) |
26.7%
(24.9 to 28.5) |
0.6% (0.3 to 1.1) |
1.0%
(0.6 to 1.5) |
10.9%
(9.7 to 12.3) |
0.3%
(0.1 to 0.6) |
6.5% (5.6 to 7.6) |
56.9% (54.9 to 59) |
||
| P values | 7.80E-25 | 0.0078 | 0.024 | 0.14 | 0.001 | 0.0043 | 6.90E-07 | 1.10E-28 | 3.8E-4 | 0.018 | 0.039 | ||
| Cannabis abuse | Cases | 14.3% (10.7 to 18.7) |
23.0% (18.6 to 28) |
– |
50.0%
(44.6 to 55.4) |
71.7%
(66.4 to 76.5) |
60.2%
(54.7 to 65.6) |
2.5% (1.2 to 5.0) |
0.6% (0.1 to 2.5) |
21.7%
(17.4 to 26.7) |
1.9%
(0.8 to 4.2) |
12.4%
(9.1 to 16.6) |
98.1%
(95.8 to 99.2) |
| Controls | 18% (16.5 to 19.5) |
16.8% (15.4 to 18.4) |
1.1%
(0.8 1.7) |
28.8%
(27.1 to 30.7) |
23.7%
(22.1 to 25.5) |
0.7% (0.4 to 1.1) |
1.6% (1.2 to 2.2) |
13.3%
(12.0 to 14.8) |
0.3%
(0.2 to 0.7) |
6.4%
(5.4 to 7.4) |
52.5%
(50.6 to 54.5) |
||
| P values | 0.12 | 0.0078 | 6.0E-233 | 2.2E-52 | 2.0E-42 | 0.0026 | 0.25 | 7.50E-05 | 0.0012 | 0.00011 | 2.80E-54 | ||
| Cannabis dependence | Cases | 14.8% (10.2 to 20.9) | 23.8% (18.1 to 30.6) |
85.2%
(79.1 89.8) |
– |
69.8%
(62.7 to 76.2) |
65.1%
(57.8 71.8) |
2.6% (1.0 to 6.4) |
0.5% (0.0 to 3.4) |
27.5%
(21.4 34.6) |
3.2%
(1.3 to 7.1) |
17.5%
(12.5 to 23.8) |
97.9%
(94.3 to 99.3) |
| Controls | 17.7% (16.3 to 19.3) |
17.1% (15.6 to 18.6) |
6.2%
(5.3 to 7.2) |
31.2%
(29.4 to 33) |
25.3%
(23.6 to 27) |
0.7% (0.5 to 1.2) |
1.6% (1.2 to 2.2) |
13.4%
(12.1 to 14.7) |
0.3%
(0.1 to 0.6) |
6.3%
(5.4 7.3) |
54.9%
(53.0 to 56.8) |
||
| P values | 0.36 | 0.024 | 6.0E-233 | 5.20E-27 | 1.50E-31 | 0.02 | 0.4 | 1.50E-07 | 1.30E-06 | 1.80E-08 | 1.80E-30 | ||
| Alcohol abuse | Cases | 18.0% (15.6 to 20.7) | 19.1% (16.6 to 21.8) | 24.6% (21.9 to 27.5) | 14.1% (11.9 to 16.5) | – | 63.3% (60.1 to 66.4) | 1.3% (0.7 to 2.3) | 1.8% (1.1 to 2.9) | 17.4% (15.0 to 20.0) | 1.2%(0.6 2.2) | 8.0% (6.4 to 10.0) | 82.3% (79.7 to 84.7) |
| Controls | 17.3% (15.6 to 19.1) | 16.7% (15.1 to 18.5) | 5.0% (4.0 to 6.1) | 3.1% (2.4 to 4.0) | 9.9% (8.6 to 11.4) | 0.7% (0.4 to 1.2) | 1.4% (0.9 to 2.0) | 12.8% (11.3 to 14.4) | 0.2%(0.0 0.5) | 6.6% (5.5 to 7.9) | 45.3% (43.0 to 47.7) | ||
| P values | 0.66 | 0.14 | 2.2E-52 | 5.2E-27 | 1.7E-192 | 0.14 | 0.45 | 0.0012 | 0.0011 | 0.2 | 2.8E-77 | ||
| Alcohol dependence | Cases | 18.8% (16.2 to 21.8) | 21.4% (18.6 to 24.5) | 25% (22.0 to 28.2) | 15.9% (13.4 to 18.7) | 76.5% (73.4 to 79.5) | – | 1.3% (0.7 to 2.4) | 1.9% (1.1 to 3.2) | 19.3% (16.6 to 22.3) | 1.2% (0.6 to 2.3) | 7.9% (6.1 to 10.0) | 83.1% (80.3 to 85.6) |
| Controls | 17% (15.4 to 18.8) | 16% (14.5 to 17.7) | 6.4%(5.4 to 7.6) | 3.3% (2.6 to 4.2) | 17.2% (15.6 to 19.0) | 0.7% (0.4 to 1.2) | 1.4% (0.9 to 2.0) | 12.4% (11.0 to 13.9) | 0.3% (0.1 to 0.6) | 6.8% (5.7 to 8.0) | 48% (45.8 to 50.2) | ||
| P values | 0.29 | 0.001 | 2.00E-42 | 1.50E-31 | 1.7E-192 | 0.2 | 0.34 | 3.50E-06 | 0.0062 | 0.35 | 4.90E-63 | ||
| Panic disorder with agoraphobia | Cases | 37.5% (19.6 to 59.2) | 41.7% (22.8 to 63.1) | 33.3% (16.4 to 55.3) | 20.8% (7.9 to 42.7) | 50% (31.4 to 68.6) | 41.7% (22.8 to 63.1) | – | NA | NA | 4.2% (0.2 to 23.1) | 4.2% (0.2 to 23.1) | 75.0% (52.9 to 89.4) |
| Controls | 17.4% (16 to 18.8) |
17.3% (15.9 to 18.8) |
11.4% (10.3 to 12.7) |
6.7% (5.8 to 7.7) |
33.7% (31.9 to 35.5) |
27.9% (26.2 to 29.6) |
1.5% (1.1 to 2.1) |
13.6% (12.3 to 14.9) |
0.5% (0.3 to 0.8) |
7.1% (6.2 to 8.1) |
57.7% (55.8 to 59.5) |
||
| P values | 0.021 | 0.0043 | 0.0026 | 0.02 | 0.14 | 0.2 | NA* | NA* | 0.27 | 0.88 | 0.13 | ||
| Panic disorder without agoraphobia | Cases |
50%
(35.5 to 64.5) |
47.6%
(32.3 to 63.4) |
4.8% (0.8 to 17.4) |
2.4% (0.1 to 14.1) |
40.5% (26.0 to 56.7) |
35.7% (22.0 to 52.0) |
0% | – | 100% | 0% | 19.0% (9.1 to 34.6) |
66.7% (50.4 to 80) |
| Controls |
17%
(15.6 to 18.5) |
17.1%
(15.7 to 18.5) |
11.7% (10.5 to 13) | 6.9% (6.0 to 7.9) |
33.7% (32.0 to 35.5) |
27.9% (26.2 to 29.6) |
0.9% (0.6 to 1.3) |
13% (11.8 to 14.3) |
0.5% (0.3 to 0.9) |
6.9% (6.0 to 7.9) |
57.7% (55.8 to 59.6) |
||
| P values | 7.70E-08 | 6.90E-07 | 0.25 | 0.4 | 0.45 | 0.34 | NA | NA | 1† | 0.006 | 0.31 | ||
| Panic attack | Cases |
38%
(33.3 to 43) |
37.3%
(32.5 to 42.3) |
17.6%
(14.1 to 21.8) |
13.1%
(10 to 16.9) |
41.1%
(36.2 to 46.1) |
37.8% (33.0 to 42.8) | 6.0% (4.0 to 9.0) |
10.6% (7.8 to 14.1) |
– |
2.3%
(1.1 to 4.4) |
15.6%
(12.3 to 19.7) |
64.7% (59.8 to 69.4) |
| Controls |
14.1%
(12.7 to 15.6) |
14.2%
(12.9 to 15.7) |
10.6%
(9.4 to 11.9) |
5.8%
(4.9 to 6.8) |
32.6%
(30.7 to 34.6) |
26.3% (24.6 to 28.2) | NA | NA |
0.2%
(0.1 to 0.5) |
5.6%
(4.8 to 6.7) |
56.7% (54.7 to 58.7) |
||
| P values | 8.30E-31 | 1.10E-28 | 7.50E-05 | 1.50E-07 | 0.0012 | 3.50E-06 | NA* | NA* | 6.70E-07 | 1.50E-12 | 0.0032 | ||
| Manic episode | Cases | 35.7% (14 to 64.4) |
57.1%
(29.6 to 81.2) |
42.9%
(18.8 to 70.4) |
42.9%
(18.8 to 70.4) |
78.6%
(48.8 to 94.3) |
64.3% (35.6 to 86) |
7.1% (0.4 to 35.8) |
0.0% | 64.3% (35.6 to 86.0) | – | 7.1% (0.4 to 35.8) |
78.6% (48.8 to 94.3) |
| Controls | 17.4% (16 to 18.9) |
17.3%
(15.9 to 18.8) |
11.5%
(10.3 to 12.7) |
6.6%
(5.7 to 7.6) |
33.6% (31.8 to 35.4) | 27.8% (26.1 to 29.5) |
0.8% (0.5 to 1.3) |
1.5% (1.1 to 2.1) |
14.1%
(12.8 to 15.4) |
7.1% (6.2 to 8.1) |
57.7% (55.9 to 59.6) |
||
| P values | 0.15 | 0.00038 | 0.0012 | 1.30E-06 | 0.0011 | 0.0062 | 0.27 | 1† | 6.70E-07 | 1 | 0.19 | ||
| Psychotic symptoms | Cases | 28.1% 22 35 | 24.0% (18.3 to 30.7) |
20.4%
(15.1 to 26.9) |
16.8%
(12.0 to 23.0) |
38.3% (31.5 to 45.5) |
31.1% (24.8 to 38.2) | 0.5% (0.0 to 3.2) |
4.1% (1.9 to 8.2) |
31.6%
(25.3 to 38.7) |
0.5% (0.0 to 3.2) |
– | 65.3% (58.1 to 71.9) |
| Controls | 16.7% (15.3 to 18.2) | 17% (15.6 to 18.6) |
10.9%
(9.8 to 12.2) |
6.1%
(5.2 to 7.1) |
33.5% (31.7 to 35.4) |
27.7% (26.0 to 29.5) |
0.9% (0.6 to 1.4) |
1.3% (0.9 to 1.9) |
13%
(11.7 to 14.4) |
0.5% (0.3 to 0.9) |
57.3% (55.3 to 59.2) |
||
| P values | 8.60E-05 | 0.018 | 1.1E-4 | 1.8E-08 | 0.2 | 0.35 | 0.88 | 0.006 | 1.5E-12 | 1 | 0.034 | ||
| Any illegal substance use‡ | Cases | 18.2% (16.4 to 20.2) |
18.8% (17.0 to 20.8) |
19.7% (17.8 to 21.8) |
11.5%
(10.0 to 13.2) |
48.1%
(45.7 to 50.6) |
40.2%
(37.8 to 42.7) |
1.1% (0.7 to 1.8) |
1.7% (1.2 to 2.5) |
16.0% (14.3 to 17.9) |
0.7% (0.4 to 1.3) |
8.0% (6.7 to 9.4) |
– |
| Controls | 16.6% (14.5 to 18.9) |
15.7% (13.7 to 18.0) |
0.5%
(0.2 to 1.2) |
0.3%
(0.1 to 0.9) |
14.2%
(12.3 to 16.4) |
11.2%
(9.5 to 13.2) |
0.5% (0.2 to 1.2) |
1.2% (0.7 to 2.1) |
12.0% (10.2 to 14.0) |
0.3% (0.1 to 0.8) |
5.8% (4.6 to 7.4) |
||
| P values | 0.29 | 0.039 | 2.80E-54 | 1.80E-30 | 2.80E-77 | 4.90E-63 | 0.13 | 0.31 | 0.0032 | 0.19 | 0.034 |
Read rows first followed by columns, for example, of participants with MDD 33.5% also had social anxiety. Significant P -values after multiple testing correction (Bonferroni corrected, significance threshold: 0.05/24=1.7E-3) appear in bold.
*Diagnoses and criteria whose definitions mutually exclude each other (eg, panic attack required for a diagnosis of panic disorder).
†Fisher’s exact test used.
‡Illicit drug or non-medical use of prescription drug. Include cocaine, amphetamine-type stimulants, inhalants, sedatives or sleeping pills, hallucinogens, opioids, party drugs (ecstasy, ketamine, GHB), over-the-counter/prescription pain killers and analgesics for non-medical purposes, Over-the-counter/prescription stimulants for non-medical purposes, or other.
MDD, major depressive disorder.
bmjopen-2017-018959supp003.pdf (23.3KB, pdf)
Consistent with previous reports,45 50 52 55 most mood disorders were significantly comorbid (table 5, online supplementary file 3) and the non-significant associations may be explained by the low prevalence of manic episodes and agoraphobia (yielding low statistical power). In addition, MDD was also associated with presence of psychotic symptoms, previously reported in an Australian sample69 and in a worldwide mega-analysis.70 An association between social anxiety and psychotic symptoms had also been reported69 70 but did not reach significance in our study. Finally, panic attacks were associated with higher risk of all affective disorders, substance abuse, dependence and initiation, as well as psychotic symptoms, most of which were already reported using the DSM-IV classification.55 Panic disorder, on the other hand, was only associated with increased risk of affective disorders and alcohol abuse/dependence in the 19Up study.55 67
Substance misuse and any drug use were, unsurprisingly,57 highly comorbid (table 5, online supplementary file 3). Furthermore, alcohol and cannabis abuse and dependence were associated with higher risk of panic attacks55 and manic episodes.73 Finally, alcohol dependence was more likely to be reported by individuals with social anxiety,74 while cannabis abuse and dependence were more common in individuals reporting psychotic symptoms.70
Strengths and limitations
The 19Up study is a major resource to study mental health and substance use in an Australian sample of young adults. The main strengths of the 19Up within the BLTS are
Large sample size (n=2773; 369 monozygotic and 494 dizygotic twin pairs): Provides significant power (>0.8) to detect heritability >0.25, shared environment influences >0.2 and a genetic correlation >0.3 (when heritability for both phenotypes>20%).75 76
Genotyping: The majority (84%) of the sample has been genotyped allowing GWAS studies,77 SNP-based heritability estimation75 78 and polygenic risk scores analyses.79 80
Longitudinal design: Most participants have been assessed at 12, 14, 16 and 21 years.
Well-characterised lifetime psychiatric and substance use, DSM-IV abuse and dependence criteria, for a wide variety of licit and illicit substances (including non-medical use of over-the-counter and prescription substances).
Rich biological samples: Hair sample (cortisol, see ref.81) and longitudinal blood samples (vitamin D, antibodies, metabolites, gene expression, GWAS).
Multimodal Imaging: 36% of participants underwent structural and functional MRI and DTI.
Repeated observations within 19Up, to study scores and diagnoses stability and reliability.
A main limitation of the 19Up study is the relatively young age of the participants when estimating lifetime prevalence as some controls may develop a mental disorder after the time of assessment. However, it is expected that a later assessment will capture additional lifetime cases. The next wave of the BLTS, currently under way, should provide a further assessment of the twins’ psychopathologies in adulthood. In addition, twins are not necessarily a random sample of the population as twinning is likely heritable82 and could be associated to some traits of interest. However, we can compare twins and non-twin siblings in this sample to rule out any confounding effect of twinning. Another limitation is that the factors influencing the different participation rates in the different waves (or in the overall study) are largely unknown. This non-random sampling could limit making inference about the general BLTS sample or the general population. Finally, the fact the assessments of clinical diagnoses were completed using different instruments (phone or online) and outside of a clinical interview with a psychiatrist or psychologist may also be a limitation.
Here, we reported the demographics of the full 19Up sample and highlighted the high lifetime prevalence and comorbidities between psychiatric disorder present in an unselected sample of young Australian adult twins. This should allow future studies to use the rich BLTS data in order to shed light on the pathways to psychiatric disorders. Finally, we hope that publicising the 19Up study (and the BLTS) may lead to new collaborations.
Acknowledgments
The authors are grateful to the twins for their generosity of time and willingness to participate in their studies. They thank Marlene Grace and Ann Eldridge for twin recruitment and data collection from 1992 to 2009, Lenore Sullivan, Lorelle Nunn, Mary Ferguson, Kerri McAloney, and Lucy Winkler for 19Up data collection, Daniel Park, David Smyth and Anthony Conciatore for IT support. They would like to thank the reviewers for their reviews and helpful comments.
Footnotes
NGM, IBH and NAG contributed equally.
BC-D and VO’C contributed equally.
Contributors: NGM, IBH, NAG, JJM, SEM, MJW, JS, BPZ and PAL designed the 19Up study. BCD, VO, RP, KMK, JS and NM processed the data. BCD and VO analysed the data and drafted the manuscript. RP, NM, KMK, JS, AV, DFH, PAL, TAD, JMB, MC, BPZ, JS, MJW, SEM, JJM, NGM IBH and NAG revised the article and agree with the final version and the findings.
Funding: The NHMRC (APP10499110) and the NIH (K99R00, R00DA023549) funded the 19Up study. The MRI was supported by grants from NIH (R01HD050735) and the NHMRC (496682, 1009064). Genotyping was funded by the NHMRC (389891).
Competing interests: BCD is supported by an UQI scholarship, VOC by a UQ winter scholarship. SEM is supported by an NHMRC fellowship (APP1103623), JGS by a NHMRC Practitioner Fellowship (1105807), JJMG by a NHMRC John Cade Fellowship (APP1056929), BPZ is supported by an ARC Discovery Early Career Research Award (DE120100562), NAG by NIH/NIDA grants (5R00DA023549, R21DA038852).
Patient consent: Obtained.
Ethics approval: QIMR Human Research and Ethics Committee and the Virginia Commonwealth University Institutional Review Board approved the study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data used in this analysis and described in this article are available to all interested researchers through collaboration. Please contact NGM (Nick.Martin@qimrberghofer.edu.au).
References
- 1. Gillespie NA, Henders AK, Davenport TA, et al. . The Brisbane Longitudinal Twin Study: Pathways to Cannabis Use, Abuse, and Dependence project-current status, preliminary results, and future directions. Twin Res Hum Genet 2013;16:21–33. 10.1017/thg.2012.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wright MJ, Martin NG. Brisbane adolescent twin study: Outline of study methods and research projects. Aust J Psychol 2004;56:65–78. [Google Scholar]
- 3. Wright MJ, Hansell NK, Geffen GM, et al. . Genetic influence on the variance in P3 amplitude and latency. Behav Genet 2001;31:555–65. [DOI] [PubMed] [Google Scholar]
- 4. Wright MJ, Luciano M, Hansell NK, et al. . Genetic sources of covariation among P3(00) and online performance variables in a delayed-response working memory task. Biol Psychol 2002;61:183–202. [DOI] [PubMed] [Google Scholar]
- 5. Luciano M, Wright M, Smith GA, et al. . Genetic covariance among measures of information processing speed, working memory, and IQ. Behav Genet 2001;31:581–92. [DOI] [PubMed] [Google Scholar]
- 6. Hansell NK, Wright MJ, Medland SE, et al. . Genetic co-morbidity between neuroticism, anxiety/depression and somatic distress in a population sample of adolescent and young adult twins. Psychol Med 2012;42:1249–60. 10.1017/S0033291711002431 [DOI] [PubMed] [Google Scholar]
- 7. Wright M, De Geus E, Ando J, et al. . Genetics of cognition: outline of a collaborative twin study. Twin Res 2001;4:48–56. [DOI] [PubMed] [Google Scholar]
- 8. Reed DR, Zhu G, Breslin PA, et al. . The perception of quinine taste intensity is associated with common genetic variants in a bitter receptor cluster on chromosome 12. Hum Mol Genet 2010;19:4278–85. 10.1093/hmg/ddq324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hwang LD, Zhu G, Breslin PA, et al. . A common genetic influence on human intensity ratings of sugars and high-potency sweeteners. Twin Res Hum Genet 2015;18:361–7. 10.1017/thg.2015.42 [DOI] [PubMed] [Google Scholar]
- 10. Zhu G, Duffy DL, Eldridge A, et al. . A major quantitative-trait locus for mole density is linked to the familial melanoma gene CDKN2A: a maximum-likelihood combined linkage and association analysis in twins and their sibs. Am J Hum Genet 1999;65:483–92. 10.1086/302494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McGregor B, Pfitzner J, Zhu G, et al. . Genetic and environmental contributions to size, color, shape, and other characteristics of melanocytic naevi in a sample of adolescent twins. Genet Epidemiol 1999;16:40–53. [DOI] [PubMed] [Google Scholar]
- 12. Shekar SN, Luciano M, Duffy DL, et al. . Genetic and environmental influences on skin pattern deterioration. J Invest Dermatol 2005;125:1119–29. 10.1111/j.0022-202X.2005.23961.x [DOI] [PubMed] [Google Scholar]
- 13. Kessler RC, Ustün TB. The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). Int J Methods Psychiatr Res 2004;13:93–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 2008;9:947–57. 10.1038/nrn2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McGorry PD, Purcell R, Goldstone S, et al. . Age of onset and timing of treatment for mental and substance use disorders: implications for preventive intervention strategies and models of care. Curr Opin Psychiatry 2011;24:301–6. 10.1097/YCO.0b013e3283477a09 [DOI] [PubMed] [Google Scholar]
- 16. Degenhardt L, Ferrari AJ, Calabria B, et al. . The global epidemiology and contribution of cannabis use and dependence to the global burden of disease: results from the GBD 2010 study. PLoS One 2013;8 10.1371/journal.pone.0076635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gillespie NA, Neale MC, Kendler KS. Pathways to cannabis abuse: a multi-stage model from cannabis availability, cannabis initiation and progression to abuse. Addiction 2009;104:430–8. 10.1111/j.1360-0443.2008.02456.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hickie IB, Davenport TA, Hadzi-Pavlovic D, et al. . Development of a simple screening tool for common mental disorders in general practice. Med J Aust 2001;175(Suppl):S10–17. [DOI] [PubMed] [Google Scholar]
- 19. Hickie IB. Primary care psychiatry is not specialist psychiatry in general practice. Med J Aust 1999;170:171–3. [PubMed] [Google Scholar]
- 20. Clarke DM, McKenzie DP. An examination of the efficiency of the 12-item SPHERE questionnaire as a screening instrument for common mental disorders in primary care. Aust N Z J Psychiatry 2003;37:236–9. 10.1046/j.1440-1614.2003.01112.x [DOI] [PubMed] [Google Scholar]
- 21. Berryman C, McAuley JH, Moseley LG. Sphere 12 screening questionnaire. J Physiother 2012;58:273 10.1016/S1836-9553(12)70133-9 [DOI] [PubMed] [Google Scholar]
- 22. Kessler RC, Andrews G, Colpe LJ, et al. . Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med 2002;32:959–76. [DOI] [PubMed] [Google Scholar]
- 23. Heatherton TF, Kozlowski LT, Frecker RC, et al. . The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 1991;86:1119–27. [DOI] [PubMed] [Google Scholar]
- 24. Swanson J, et al. . Categorical and dimensional definitions and evaluations of symptoms of ADHD: The snap and the swan ratings scales. 2005;2014 http://www.adhd.net/SNAP_SWAN.pdf [PMC free article] [PubMed] [Google Scholar]
- 25. Altman EG, Hedeker D, Peterson JL, et al. . The Altman Self-Rating Mania Scale. Biol Psychiatry 1997;42:948–55. 10.1016/S0006-3223(96)00548-3 [DOI] [PubMed] [Google Scholar]
- 26. Buysse DJ, Reynolds CF, Monk TH, et al. . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 27. Morin CM, Belleville G, Bélanger L, et al. . The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011;34:601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parker G, Tupling H, Brown LB. A Parental Bonding Instrument. British Journal of Medical Psychology 1979;52:1–10. [Google Scholar]
- 29. Brugha T, Bebbington P, Tennant C, et al. . The List of Threatening Experiences: a subset of 12 life event categories with considerable long-term contextual threat. Psychol Med 1985;15:189–94. [DOI] [PubMed] [Google Scholar]
- 30. Brugha TS, Cragg D. The List of Threatening Experiences: the reliability and validity of a brief life events questionnaire. Acta Psychiatr Scand 1990;82:77–81. [DOI] [PubMed] [Google Scholar]
- 31. Rosmalen JG, Bos EH, de Jonge P. Validation of the Long-term Difficulties Inventory (LDI) and the List of Threatening Experiences (LTE) as measures of stress in epidemiological population-based cohort studies. Psychol Med 2012;42:2599–608. 10.1017/S0033291712000608 [DOI] [PubMed] [Google Scholar]
- 32. Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Questionnaire (Junior and Adult). (Hodder & Stoughton 1975. [Google Scholar]
- 33. Costa PT, McCrae RR. The NEO personality inventory manual: Psychological Assessment Resources, 1985. [Google Scholar]
- 34. Morey LC. Personality assessment inventory, professional manual: Psychological Assessment Resources, Inc, 1991. [Google Scholar]
- 35. Schmaal L, Hibar DP, Sämann PG, et al. . Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry 2017;22 10.1038/mp.2016.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schmaal L, Veltman DJ, van Erp TG, et al. . Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry 2016;21:806–12. 10.1038/mp.2015.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sudmant PH, Rausch T, Gardner EJ, et al. . An integrated map of structural variation in 2,504 human genomes. Nature 2015;526:75–81. 10.1038/nature15394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Auton A, Brooks LD, Durbin RM, et al. . A global reference for human genetic variation. Nature 2015;526:68–74. 10.1038/nature15393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McCarthy S, Das S, Kretzschmar W, et al. . A reference panel of 64,976 haplotypes for genotype imputation. Nat Genet 2016;48 10.1038/ng.3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang J, Bakshi A, Zhu Z, et al. . Genetic variance estimation with imputed variants finds negligible missing heritability for human height and body mass index. Nat Genet 2015;47:1114–20. 10.1038/ng.3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Zubicaray GI, Chiang MC, McMahon KL, et al. . Meeting the Challenges of Neuroimaging Genetics. Brain Imaging Behav 2008;2:258–63. 10.1007/s11682-008-9029-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miller SM, Hansell NK, Ngo TT, et al. . Genetic contribution to individual variation in binocular rivalry rate. Proc Natl Acad Sci U S A 2010;107:2664–8. 10.1073/pnas.0912149107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Slade T, Johnston A, Oakley Browne MA, et al. . 2007 National Survey of Mental Health and Wellbeing: methods and key findings. Aust N Z J Psychiatry 2009;43:594-605 10.1080/00048670902970882 [DOI] [PubMed] [Google Scholar]
- 44. Kessler RC, Sampson NA, Berglund P, et al. . Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci 2015;24:210–26. 10.1017/S2045796015000189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Merikangas KR, He JP, Burstein M, et al. . Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry 2010;49:980–9. 10.1016/j.jaac.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bromet E, Andrade LH, Hwang I, et al. . Cross-national epidemiology of DSM-IV major depressive episode. BMC Med 2011;9:90 10.1186/1741-7015-9-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oakley Browne MA, Wells JE, Scott KM, et al. . Lifetime prevalence and projected lifetime risk of DSM-IV disorders in Te Rau Hinengaro: the New Zealand Mental Health Survey. Aust N Z J Psychiatry 2006;40:865–74. 10.1080/j.1440-1614.2006.01905.x [DOI] [PubMed] [Google Scholar]
- 48. Williams L, Jacka F, Pasco J, et al. . The prevalence of mood and anxiety disorders in Australian women. Australas Psychiatry 2010;18:250–5. 10.3109/10398561003731155 [DOI] [PubMed] [Google Scholar]
- 49. McEvoy PM, Grove R, Slade T. Epidemiology of anxiety disorders in the Australian general population: findings of the 2007 Australian National Survey of Mental Health and Wellbeing. Aust N Z J Psychiatry 2011;45:957–67. 10.3109/00048674.2011.624083 [DOI] [PubMed] [Google Scholar]
- 50. Kessler RC, Amminger GP, Aguilar-Gaxiola S, et al. . Age of onset of mental disorders: a review of recent literature. Curr Opin Psychiatry 2007;20:359–64. 10.1097/YCO.0b013e32816ebc8c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Crome E, Grove R, Baillie AJ, et al. . DSM-IV and DSM-5 social anxiety disorder in the Australian community. Aust N Z J Psychiatry 2015;49:227–35. 10.1177/0004867414546699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sanders AR, Levinson DF, Duan J, et al. . The Internet-based MGS2 control sample: self report of mental illness. Am J Psychiatry 2010;167:854–65. 10.1176/appi.ajp.2010.09071050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. López-Solà C, Fontenelle LF, Alonso P, et al. . Prevalence and heritability of obsessive-compulsive spectrum and anxiety disorder symptoms: A survey of the Australian Twin Registry. Am J Med Genet B Neuropsychiatr Genet 2014;165B:314–25. 10.1002/ajmg.b.32233 [DOI] [PubMed] [Google Scholar]
- 54. Kessler RC, Chiu WT, Jin R, et al. . The epidemiology of panic attacks, panic disorder, and agoraphobia in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2006;63:415–24. 10.1001/archpsyc.63.4.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mewton L, Slade T, Teesson M. An evaluation of the proposed DSM-5 cannabis use disorder criteria using Australian national survey data. J Stud Alcohol Drugs 2013;74:614–21. [DOI] [PubMed] [Google Scholar]
- 56. Teesson M, Slade T, Swift W, et al. . Prevalence, correlates and comorbidity of DSM-IV Cannabis Use and Cannabis Use Disorders in Australia. Aust N Z J Psychiatry 2012;46:1182–92. 10.1177/0004867412460591 [DOI] [PubMed] [Google Scholar]
- 57. Knopik VS, Heath AC, Madden PA, et al. . Genetic effects on alcohol dependence risk: re-evaluating the importance of psychiatric and other heritable risk factors. Psychol Med 2004;34:1519–30. [DOI] [PubMed] [Google Scholar]
- 58. Welfare, A. I. o. H. a. Drug statistics series no. 27. Canberraz: AIHW, 2011. [Google Scholar]
- 59. McGrath JJ, Saha S, Al-Hamzawi A, et al. . Psychotic Experiences in the General Population: A Cross-National Analysis Based on 31,261 Respondents From 18 Countries. JAMA Psychiatry 2015;72:697–705. 10.1001/jamapsychiatry.2015.0575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Linscott RJ, van Os J. An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med 2013;43:1133–49. 10.1017/S0033291712001626 [DOI] [PubMed] [Google Scholar]
- 61. Johns LC, Cannon M, Singleton N, et al. . Prevalence and correlates of self-reported psychotic symptoms in the British population. Br J Psychiatry 2004;185:298–305. 10.1192/bjp.185.4.298 [DOI] [PubMed] [Google Scholar]
- 62. Scott J, Chant D, Andrews G, et al. . Psychotic-like experiences in the general community: the correlates of CIDI psychosis screen items in an Australian sample. Psychol Med 2006;36:231–8. 10.1017/S0033291705006392 [DOI] [PubMed] [Google Scholar]
- 63. Morgan VA, Waterreus A, Jablensky A, et al. . People living with psychotic illness in 2010: the second Australian national survey of psychosis. Aust N Z J Psychiatry 2012;46:735–52. 10.1177/0004867412449877 [DOI] [PubMed] [Google Scholar]
- 64. Goldney RD, Eckert KA, Hawthorne G, et al. . Changes in the prevalence of major depression in an Australian community sample between 1998 and 2008. Aust Nz J Psychiat 2010;44:901–10. 10.3109/00048674.2010.490520 [DOI] [PubMed] [Google Scholar]
- 65. Slade T, Johnston A, Oakley Browne MA, et al. . 2007 National Survey of Mental Health and Wellbeing: methods and key findings. Aust N Z J Psychiatry 2009;43:594–605. 10.1080/00048670902970882 [DOI] [PubMed] [Google Scholar]
- 66. Starcevic V, Latas M, Kolar D, et al. . Co-occurrence of Axis I and Axis II disorders in female and male patients with panic disorder with agoraphobia. Compr Psychiatry 2008;49:537–43. 10.1016/j.comppsych.2008.02.009 [DOI] [PubMed] [Google Scholar]
- 67. Lai HM, Cleary M, Sitharthan T, et al. . Prevalence of comorbid substance use, anxiety and mood disorders in epidemiological surveys, 1990-2014: A systematic review and meta-analysis. Drug Alcohol Depend 2015;154:1–13. 10.1016/j.drugalcdep.2015.05.031 [DOI] [PubMed] [Google Scholar]
- 68. Varghese D, Scott J, Welham J, et al. . Psychotic-like experiences in major depression and anxiety disorders: a population-based survey in young adults. Schizophr Bull 2011;37:389–93. 10.1093/schbul/sbp083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. McGrath JJ, Saha S, Al-Hamzawi A, et al. . The Bidirectional Associations Between Psychotic Experiences and DSM-IV Mental Disorders. Am J Psychiatry 2016;173 10.1176/appi.ajp.2016.15101293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bulik-Sullivan B, Finucane HK, Anttila V, et al. . An atlas of genetic correlations across human diseases and traits. Nat Genet 2015;47:1236–41. 10.1038/ng.3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lee SH, Ripke S, Neale BM, et al. . Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet 2013;45:984 10.1038/ng.2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Quello SB, Brady KT, Sonne SC. Mood disorders and substance use disorder: a complex comorbidity. Sci Pract Perspect 2005;3:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Buckner JD, Schmidt NB, Lang AR, et al. . Specificity of social anxiety disorder as a risk factor for alcohol and cannabis dependence. J Psychiatr Res 2008;42:230–9. 10.1016/j.jpsychires.2007.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Neale MC, Cardon LR. Methodology for genetic studies of twins and families: Kluwer Academic Pub, 1992. [Google Scholar]
- 75. Martin NG, Eaves LJ, Kearsey MJ, et al. . The power of the classical twin study. Heredity 1978;40:97–116. [DOI] [PubMed] [Google Scholar]
- 76. Visscher PM, Andrew T, Nyholt DR. Genome-wide association studies of quantitative traits with related individuals: little (power) lost but much to be gained. Eur J Hum Genet 2008;16:387–90. 10.1038/sj.ejhg.5201990 [DOI] [PubMed] [Google Scholar]
- 77. Yang J, Lee SH, Goddard ME, et al. . GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet 2011;88:76–82. 10.1016/j.ajhg.2010.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wray NR, Goddard ME, Visscher PM. Prediction of individual genetic risk of complex disease. Curr Opin Genet Dev 2008;18:257–63. 10.1016/j.gde.2008.07.006 [DOI] [PubMed] [Google Scholar]
- 79. Wray NR, Lee SH, Mehta D, et al. . Research review: Polygenic methods and their application to psychiatric traits. J Child Psychol Psychiatry 2014;55:1068–87. 10.1111/jcpp.12295 [DOI] [PubMed] [Google Scholar]
- 80. Rietschel L, Streit F, Zhu G, et al. . Hair Cortisol and Its Association With Psychological Risk Factors for Psychiatric Disorders: A Pilot Study in Adolescent Twins. Twin Res Hum Genet 2016. 19 10.1017/thg.2016.50 [DOI] [PubMed] [Google Scholar]
- 81. Mbarek H, Steinberg S, Nyholt DR, et al. . Identification of Common Genetic Variants Influencing Spontaneous Dizygotic Twinning and Female Fertility. Am J Hum Genet 2016;98:898–908. 10.1016/j.ajhg.2016.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-018959supp002.pdf (30.9KB, pdf)
bmjopen-2017-018959supp001.pdf (48.3KB, pdf)
bmjopen-2017-018959supp003.pdf (23.3KB, pdf)