Abstract
Glucocorticoids (GCs) are essential steroid hormones that regulate numerous metabolic and homeostatic functions in almost all physiological systems. Synthetic glucocorticoids are among the most commonly prescribed drugs for the treatment of various conditions including autoimmune, allergic and inflammatory diseases. Glucocorticoids are mainly used for their potent anti-inflammatory and immunosuppressive activities mediated through signal transduction by their nuclear receptor, the glucocorticoid receptor (GR). Emerging evidence showing that diverse physiological and therapeutic actions of glucocorticoids are tissue-, cell-, and sex-specific, suggests more complex actions of glucocorticoids than previously anticipated. While several synthetic glucocorticoids are widely used in the ophthalmology clinic for the treatment of several ocular diseases, little is yet known about the mechanism of glucocorticoid signaling in different layers of the eye. GR has been shown to be expressed in different cell types of the eye such as cornea, lens, and retina, suggesting an important role of GR signaling in the physiology of these ocular tissues. In this review, we provide an update on the recent findings from in vitro and in vivo studies reported in the last 5 years that aims at understanding the role of GR signaling specifically in the eye. Advances in studying the physiological effects of glucocorticoid in the eye are vital for the elaboration of optimized and targeted GCs therapies with potent anti-inflammatory potential while minimizing adverse effects.
Keywords: Glucocorticoid, Eye, Ocular diseases, Glucocorticoid receptor, Glucocorticoid signaling
Introduction
Glucocorticoids as a therapeutic approach in ophthalmology
Even after 60 years of the first introduction of glucocorticoid therapy into clinical practice, glucocorticoids (GCs) are still the most commonly prescribed drugs worldwide. Their potent anti-inflammatory and immunosuppressive capabilities made them the physician’s preferred choice for the treatment of several dermatological, autoimmune, and ocular diseases [1]. Unfortunately, the systemic use of GCs is sometimes limited by their undesirable effects associated with high doses and/or prolonged treatment. Side effects such as metabolic abnormalities, susceptibility to infections, hypertension, peptic ulcer, cataract and water/electrolyte imbalance, would eventually necessitate the discontinuation of the drug use despite its effectiveness [2].
GCs are widely used in ophthalmology as an initial as well as adjuvant therapy for the management of a diverse array of acute and chronic ocular inflammatory conditions (Table 1). Moreover, GCs are also used, either alone or in combination with antiangiogenic therapy, to inhibit pathological ocular neovascularization that might lead to vision loss if left untreated. Targeting ocular diseases has the advantage of localized treatment possibilities through the use of topical applications such as eye drops and ointments, or through intravitreal injections to mainly target the back of the eye. However, certain chronic, visually debilitating ocular diseases such as bilateral uveitis, necessitate the use of systemic corticosteroids [3], which, as expected, would demand the restriction of GCs to short-term administration [4].
Table 1.
Examples of ocular diseases treated with glucocorticoids
| Route of administration |
Commonly used glucocorticoids | Example diseases |
|---|---|---|
|
| ||
| Topical | Prednisolone acetate 0.12% – 1% | Keratitis |
| Dexamethasone phosphate 0.1% | Allergic conjunctivitis | |
| Hydrocortisone acetate 2.5% | Postoperative treatment | |
| Flurometholone 0.1% – 0.25% | Anterior uveitis | |
|
| ||
| Periocular injections | Triamcinolone acetanoid 4mg/ml | Resistant anterior uveitis |
| Methyl prednisolone acetate 80mg/ml | Intermediate uveitis | |
| Unilateral cystoid macular edema (CME) | ||
|
| ||
| Intravitreal injections | Triamcinolone acetanoid 4mg/ml | Uveitis |
| Dexamethasone 0.4mg/0.1ml | Diabetic macular edema (DME) | |
| Neovascular age-related macular degeneration | ||
| Proliferative diabetic retinopathy (PDR) | ||
|
| ||
| Intraocular implants | Flucinolone acetanoid | chronic posterior uveitis |
| Dexamethasone | Retinal vascular occlusions (RVO) | |
| Triamcinolone acetanoid | DME | |
|
| ||
| Systemic | Prednisone 1mg/kg/day | Tuberculosis uveitis |
| Prednisolone 1mg/kg/day | Thyroid eye disease | |
| Optic neuritis | ||
| Severe corneal graft rejections | ||
In the ophthalmology practice, triamcinolone acetanoid (TA), dexamethasone (DEX), prednisolone and flucinolone acetanoid (FA) are most commonly used for being potent and selective GCs [5]. Therapeutic effects of GCs are mediated through genomic actions via the glucocorticoid receptor (GR) located in the cytosol [6]. The ubiquitous expression of GR, suggests a crucial role of glucocorticoid signaling in all cells. Diverse actions of glucocorticoid signaling have been shown to be indeed cell type-specific. Moreover, recent animal studies demonstrated sexual dimorphic effect of GCs, in which DEX induced different anti-inflammatory responses in male versus female rodents [7]. Additional studies revealed sexual dimorphism of GCs in the differential gene expression profile in males and females as well as in the direction in which genes are regulated, with certain genes being induced in male and repressed in female rodents and vice versa [8, 9]. Despite the extensive use of GCs in ophthalmology, and the documentation of the expression of GR in different ocular tissues and cell types, little is yet known about the mechanism of GR signaling in the eye that is responsible for their physiological, therapeutic and/or side effects. Thus, there is an unmet need for a clear understanding of the mechanism of glucocorticoid signaling in a cell, tissue and sex-specific fashion.
Glucocorticoid receptors structure and mechanism
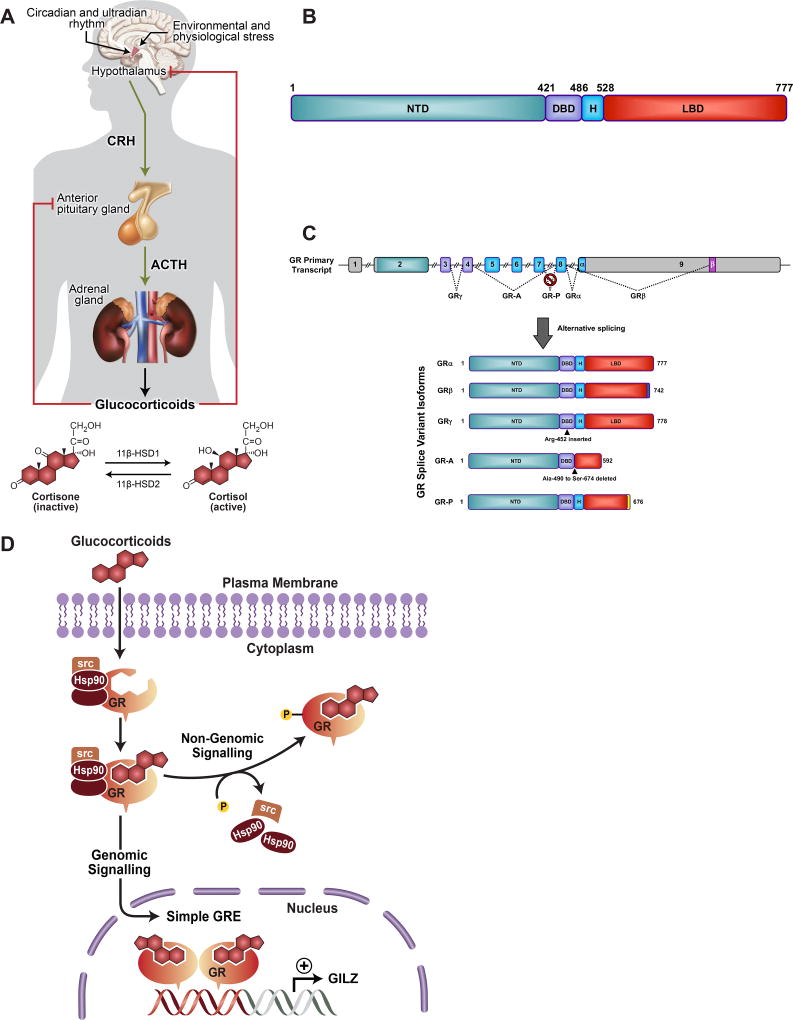
Glucocorticoids are hormones of the steroid family that are synthesized and released by the adrenal gland to play a vital role in controlling stress. The adrenal production of GCs is regulated by the hypothalamic-pituitary adrenal axis (HPA) in a circadian manner [10]. In response to a stressful stimulus, the hypothalamus releases corticotrophin-releasing hormone (CRH), which acts on the pituitary gland to stimulate the synthesis of adrenocorticotrophic hormone (ACTH) (Figure 1A). ACTH in turns activates the adrenal cortex to synthesize and release GCs, which act in cell-specific fashion in almost all body organs [11]. In humans, the biologically active form of GCs is cortisol, while corticosterone is the active form of GCs in rodents. Endogenous glucocorticoid activity is regulated at the cell level by 11β-hydroxysteroid dehydrogenase enzymes; 11β-HSD1 and 11β-HSD2, that regulate the conversion between the bioactive cortisol and its inactive precursor cortisone. Subsequently, the released GCs then act on the hypothalamus and the pituitary gland to reduce excess activation of the HPA axis as a negative feedback regulation. Disturbances in GC homeostasis result in various pathological conditions that lead to immunological and metabolic complications, Addison’s disease (deficient glucocorticoids) and Cushing’s syndrome (excessive glucocorticoids).
Figure 1.
Glucocorticoid receptor (GR) regulation and signaling. (A) Schematic representation of regulation of glucocorticoid (GC) levels by the hypothalamic-pituitary adrenal (HPA) axis, and the regulation of the conversion between the biologically active form of GCs, cortisol and the inactive form, cortisone, by 11β-hydroxysteroid dehydrogenase type 1 and type 2. (B) Representation of GR structure containing N-terminal transactivation domain (NTD), a central DNA binding domain (DBD), a flexible hinge region (H) and a C-terminal ligand binding domain (LBD). (C) GR isoforms generated by alternative splicing. (D) GR signaling following the binding of GCs to the cytoplasmic GR, which then undergoes conformational changes that result in the dissociation from accessory proteins and translocation to the nucleus. In the nucleus, GR regulates the expression of target genes by multiple ways such as a direct binding to glucocorticoid-response elements (GRE) and activation of glucocorticoid-induced leucine zipper (GILZ) transcription factor.
GCs effects are mediated by GR; a ligand-induced transcription factor and a member of the nuclear receptor family encoded by NR3C1 gene and contains 9 exons. In terms of structure, GR contains 3 major functional regions: the N-terminal transactivation domain (NTD), the central DNA binding domain (DBD) and the C-terminal ligand-binding domain (LBD) (Figure 1B). Interestingly, while a single gene encodes human GR, alternative splicing and the presence of different translation initiation sites results in a potential combination of about 40 distinct GR isoforms [12]. The existence of multiple GR isoforms is associated with varying tissue distribution and downstream signaling mechanisms. The classic cytoplasmic GC receptor is glucocorticoid receptor-α (GRα), which mediates most of GCs therapeutic and undesirable metabolic effects (Figure 1C). The splice variant GRβ, in the presence of GRα, does not bind GCs or regulate glucocorticoid-responsive reporter genes, and acts mainly as a dominant negative inhibitor of GRα activity on many GC-responsive target genes. Therefore, elevated GRβ has been associated with systemic resistance to GCs. Apart from the antagonistic effects of GRβ on GRα; GRβ has been shown to regulate a large set of genes that are not controlled by GRα, in response to the GR antagonist RU-486 [13]. Additionally, GRβ demonstrated intrinsic, GRα-independent, gene-specific transcriptional activity in the absence of a ligand, suggesting that at least a fraction of GRβ is present in the nucleus in a ligand-independent manner [14, 15]. Other GR splice variants and translational isoforms have been previously reported in different organs [12], while their physiological functions in the eye are not yet clear.
Under basal conditions, GR resides in the cytosol of cells as a part of a large multiprotein complex that includes chaperon proteins (HSP90, HSP70, P23) and immunophilins (FKBP4, FKBP5). Following ligand binding of either synthetic or endogenous GCs, GR undergoes conformational changes to dissociate from the heterocomplex, exposing nuclear localization signal and translocate to the nucleus (Figure 1D). Once in the nucleus, liganded GR dimerizes and binds to glucocorticoid response elements (GREs) to control the expression of target genes [16]. Activated GR can lead to transactivation or transrepression of gene transcription directly by binding to GREs in regulatory regions of specific target genes [17]. Another mechanism by which GR can control gene expression is through protein-protein interactions with other transcription factors. For example, majority of the therapeutic anti-inflammatory effects of GCs arise from the interactions with and subsequent antagonism of nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) and activating protein-1 (AP-1) [16]. Additionally, GR is known to regulate gene expression indirectly by binding to tethering or composite GRE [18]. GR directly binds to the p65 subunit of NF-κB and the Jun subunit of AP-1 and interferes with their transcriptional activation. Furthermore, GR has been proposed to exert rapid non-genomic actions that do not require de novo protein synthesis, by directly modulating signal transduction pathways [19]. This process occurs when cytosolic or membrane-bound GR physically interacts with kinases such as extracellular signal-regulated kinases (ERKs), mitogen activated protein kinases (MAPKs) and the p38 isoforms (p38) [20].
The eye as an immune privileged organ
The eye is a unique organ that has a direct environmental contact through the cornea and is linked to the nervous system through the retina. Additionally, the eye has a special relationship with the immune system known as immune privilege, maintained by multiple factors including ocular immunosuppressive factors, and the efficient blood-retinal barrier mainly by the tight junctions in retinal pigment epithelium (RPE) [21]. An indication of the ocular immune privilege is emphasized in corneal transplantation, with up to 90% success rate in corneal allografts without the need of systemic immunosuppressive therapy [22]. The avascular characteristic of certain ocular compartments, including cornea, lens and the outer retina, is another important anatomical feature to satisfy normal visual function and significantly contribute to the immune privilege properties of the eye. Therefore, in healthy adults, the ocular vasculature is mainly quiescent by the tight control of the balance between proangiogenic and antiangiogenic factors [23]. The abnormal growth of new blood vessels in avascular areas, such as in corneal or retinal neovascularization, or in leaky vascular compartments such as in choroidal neovascularization, interferes with their normal visual function in regulating light transmission. This will lead to several diseases including corneal opacity, proliferative diabetic retinopathy, and neovascular age-related macular degeneration (AMD) [24].
Topical administration of GCs mainly in the form of eye drops is in use for managing inflammatory insults on the ocular surface such as post-surgical inflammation and allergic conjunctivitis [25]. On the other hand, intravitreal injection of GCs is the commonly used route of administration in ophthalmology clinics for the treatment of ocular diseases in the posterior part of the eye. Due to their ability to control intraocular inflammation, reduce edema and inhibit neovascularization, intravitreal GCs are widely used for the management of sight-threatening diseases such as macular edema, macular degeneration and uveitis. TA is a frequently used glucocorticoid due to its limited solubility in the vitreous humor that minimizes its clearance and thereby provides a sustained effect [26]. TA has been shown to decrease vascular leakage and macular edema after a single administration in patients with neovascular AMD and diabetic macular edema, respectively [27]. DEX is the most potent glucocorticoid used in ophthalmology; a single dose of 0.18 mg/ml of DEX is equivalent in efficacy to 1 mg/ml of TA [28]. However, it is mostly administered in a pulse form due to its short acting effect and faster clearance from the vitreous [26]. Additionally, in diseases such as neovascular AMD, a combination therapy of GCs with the standard-of-care antiangiogenic treatments is currently being used in patients not responding to the typical therapy alone [29]. The safety of topical and intravitreal administration of GCs is somewhat controversial depending on the form of glucocorticoid used, doses, duration of treatment and patients’ predisposition. Ocular side effects of GCs might include cataract and elevation of intraocular pressure (IOP) that, if uncontrolled, may lead to glaucoma [30, 31]. Novel delivery approaches of sustained-release intravitreal GC implants are emerging to avoid the use of repeated injections and benefit patients who are resistant to conservative therapy [26]. While recent studies have demonstrated the efficiency of DEX intravitreal implants in patients with macular edema, they also pointed out to the potential increase in the frequency of IOP and cataract in those patients from the prolonged GC treatment [32]. Therefore, a clear understanding of the implications of glucocorticoid functions in normal physiology as well as their signaling mechanisms in anterior and posterior parts of the eye is critical to improve GCs benefits and minimize their undesirable effects.
Actions of glucocorticoids in the eye
Cornea
The cornea is a transparent part of the eye that covers the anterior chamber and forms a physical barrier between the eye and external agents. GCs have been shown to regulate corneal immune response, suppress inflammation and inhibit growth of blood and lymphatic vessels [33, 34]. Prednisolone, a synthetic GC analog that is commonly used in the clinic for treatment of ocular surface diseases, suppressed the expression of pro-inflammatory cytokines, induced the production of mitochondrial reactive oxygen species and thereby increased apoptosis of primary culture of human corneas as well as in a murine model of dry eye disease [35]. Transcriptome analysis of human corneal epithelial cell line (HCEC) after short-term DEX treatment elucidated significant changes in genes regulating cell movement, cytoskeletal remodeling and permeability [36]. Topical DEX treatment indeed was found to preserve corneal clarity and decrease corneal wound healing in a murine model of alkali burned cornea associated with dry eye [37]. DEX significantly reduced the expression of matrix metalloproteases (MMPs) such as MMP1, 3 and 9 as well as interleukin-6 (IL-6) while it increased the expression levels of MMP8, a collagen cleaving enzyme that plays an important role in inflammation and tissue remodeling [38]. These DEX-mediated protective effects were lost in MMP8 knockout mouse model or by using a specific MMP8 inhibitor, suggesting a crucial role of MMP8 in DEX-mediated protection in the cornea. Due to the high expression of 11β-HSD1 in corneal cells, endogenous production of cortisol has been proposed to significantly contribute to ocular surface renewal and barrier function [39]. Additionally, comparing cortisol levels in ocular surface and the serum suggested that ocular cortisol levels are potentially independent of circulatory cortisol [40]. Toll-like receptor (TLR) pathway, an important player in the recognition of pathogens, has been shown to affect cytokine but not cortisol production by HCEC, indicating that intracrine cortisol contributes to resolution of local inflammatory responses during immune-mediated challenges thereby maintaining optical clarity. Together, these studies indicate a complex role of endogenously produced and exogenously administered GCs in regulating corneal functions that is yet to be investigated in relevant in vivo models.
Lens
The lens is a transparent, biconvex structure in the eye that focuses the light coming through the cornea on the retina. In some patients, GCs have been associated with cataract formation following treatment of ocular and non-ocular diseases. However, the mechanism and pathogenesis of GC-induced cataract are not completely clear. Active GR were found in mouse and human lens epithelial cells, suggesting a direct mechanism of action of GCs on the lens. In vitro studies using human lens epithelial cells (LEC) suggested that GCs affect cell proliferation, differentiation, apoptosis, survival, and migration, through non-genomic modulation of ERK/MAPK pathway [41]. Microarray analysis of LECs revealed multiple genes that were differentially regulated following DEX treatment compared to the control groups including upregulation in monocyte chemotactic protein-1 gene; CCL2 and downregulation of FAS receptor levels, an important cell surface receptor of the tumor necrosis factor receptor family, suggesting a potential role of inflammation in GC-induced cataract [42]. This finding was further supported by another study documenting that CCL2 was detected in patients’ samples following cataract surgery [43]. The transcription factor c-Jun has been shown to bind to the promoter regions of CCL2 and FAS genes and regulate their expression. Whether c-Jun is the critical transcription factor responsible for the changes in the expression of these genes in response to GCs is not yet clear. If this is indeed the case, c-Jun could represent a potential therapeutic target to treat GC-induced cataract, which is awaiting further investigation. DEX treatment of rat lens epithelial explant has been shown to enhance fibroblast growth factor (FGF)-induced lens proliferation but retard their fiber differentiation and elongation possibly through genomic modulation of ERK1/2 activity [44]. This imbalance of relationship between proliferation and differentiation could lead to abnormal cell behavior and lens opacification. It is important to mention that the reported GC-induced cataract have been particularly linked to exogenous GC administration. Patients with Cushing’s syndrome, which is characterized by elevated levels of endogenous GCs, do not usually develop steroid-induced cataract. This observation has been largely attributed to a normal GR down-regulation mechanism that might be impaired with exogenous steroid therapy [45]. Further analyses of GC-mediated signaling in the lens and the causative players in cataract formation are essential to treat and/or prevent this undesirable adverse effect of GCs.
Trabecular meshwork
Trabecular meshwork (TM), located in the anterior chamber of the eye, adjusts aqueous humor resistance and thereby regulates IOP [46]. Within 4–6 weeks of topical ocular administration of GCs, about 40% of patient population develop ocular hypertension and secondary glaucoma [47]. These side effects are usually observed in patients with predisposing risk factors including a personal or family history of glaucoma, pre-existing type I diabetes mellitus, and connective-tissue disease, which in turn requires diligent follow-up of those patients when receiving GCs [31]. Many of GC-induced changes in TM of human eyes can be mimicked in cultured TM cells, indicating a direct action of GCs on TM [48]. Transcriptomic and proteomic analysis of DEX-treated cultured human and bovine TM cells revealed alteration in hundreds of genes and proteins involved in cell metabolism, signal transduction, cell structure/motility, intracellular traffic, and immunity [49–51]. Moreover, DEX induced the upregulation of noncanonical Wnt ligand Wnt5a, which in turn induced cross-linked actin network (CLAN) formation in cultured TM cells through RhoA/ Rho-associated protein kinases (ROCK) [52–54]. Prolonged DEX treatment of cultured TM cells increased the production and deposition of fibronectin, glycosaminoglycan and extracellular matrix (ECM) resulting in decreased outflow facility [55, 56]. Moreover, topical ocular treatment with DEX in wild-type mice induced IOP and glaucoma, and increased formation of ECM, actin, and mutant myocilin (MYOC) proteins, which has been shown to induce endoplasmic reticulum stress [57, 58], and these effects were reversed after DEX withdrawal. Interestingly, variability in patients’ responsiveness to GC-induced ocular side effects has been attributed to a lower GRβ-GRα ratio compared to their normal counterparts. Lower GRβ expression in the TM of glaucoma patients is associated with increased GCs responsiveness, and subsequently more ECM buildup and cytoskeletal changes [59]. Thus, manipulating GRβ-GRα ratio could be exploited for therapeutic intervention as a way to modulate GC sensitivity.
Retina
Retina is the neurosensory component of the eye that sends neural signals of light to the brain for visual recognition. Neural retina consists of different cell types such as photoreceptors- the light sensitive part of the retina that converts captured photons into a nerve signal through phototransduction, and Müller glial cells (MGC)- the main glial cells of the retina that play a crucial role in maintaining local environment in the retina for optimal visual function. Retinal blood vessels lined by microvascular endothelial cells sustain tissue homeostasis at steady state and undergo angiogenesis and regeneration during tissue repair.
Ocular pathologies of the retina are commonly treated with GCs either alone or in combination with other drugs. Intravitreal injections of GCs have been demonstrated in multiple studies in rodent animals as well as in humans to effectively reduce ocular neovascularization, and suppress inflammation. However, the exact mechanism by which GCs mediate these effects in the retina has not been clearly determined. Glucocorticoid-induced leucine zipper (GILZ) transcription factor was found to be down-regulated in a rat model of retinal inflammation, in the aqueous humor of human eyes with bacterial endophthalmitis [60], and in lipopolysaccharide (LPS)-treated rat retinal endothelial cells [61], underlying GCs responsiveness in retinal inflammatory diseases. GILZ overexpression suppressed LPS-induced activation of NF-κB and the retinal toll-like receptor4-myeloid differentiation factor 88 (TLR4-MyD88) signaling pathways, which has been shown to play a vital role in the inflammatory response [60]. Intravitreal TA injections in an early diabetic rat model significantly activated GR and p38 MAPK signaling pathway, thereby exerted an anti-apoptotic and protective effect on retinal neurons [62]. This finding indicates a dual beneficial potential of GCs not only as an anti-inflammatory but also as a neuroprotective treatment compared to the traditional therapy for diabetic retinopathy patients that targets the vascular endothelial growth factor (VEGF), a dominant angiogenic mediator, using biologics. Moreover, in a murine model of endotoxin-induced uveitis (EIU), DEX treatment significantly suppressed S100A8; a calcium-binding protein that has a critical role in the inflammatory and immune response, thus inhibiting the migration and infiltration of inflammatory cells from the blood [63]. Different GCs exhibited similar yet distinct effects on cytokine expression, neuroprotection, and oxidative stress pathways. Therefore, detailed analysis and understanding of the effects of individual GCs specifically in the retina is crucial to provide customized GC treatments to target specific diseases or even certain stages of each disease.
In rodent, the partial GR antagonist, mifepristone significantly increased cleaved caspase 3 levels and induced depletion of BCL-XL, the antiapoptotic molecule preferentially expressed in central and retinal neurons, which ultimately resulted in photoreceptor cell death. Moreover, DEX treatment significantly increased retinal BCL-XL, and eventually prevented mifepristone-induced photoreceptor cell death, suggesting that GC may protect retinal function and morphology by preventing photoreceptors apoptosis [64]. This suggests a potential role of GC in photoreceptor homeostasis that needs further confirmation. In human retinal endothelial cells (HRECs), cells that line the microvascular capillary beds in the retina, DEX significantly repaired glucose-induced cell loss and enhanced permeability, suggesting a potential role of DEX in protecting HRECs in high glucose condition and facilitating endothelial cell repair in the diabetic retina [65]. Additionally, DEX treatment of HRECs have been shown to suppress p38 MAPK and subsequent NF-κB signaling pathways that contribute to the regulation of claudin-5 and occludin expression, which finally strengthens the barrier function of endothelial tight junction [65, 66]. Despite showing that microvascular retinal endothelial cells are more resistant to GC-induced cell death compared to endothelial cells from other organ, they are still susceptible to GC-induced toxicity at high doses [67]. These findings strongly indicate the importance of titrating GC doses in preclinical as well as clinical studies to achieve the desired therapeutic potential while avoiding possible adverse effects. GR have been shown to be highly conserved and expressed in the MGC of retinas from different vertebrates such as zebrafish, mice, guinea pigs, and dogs [68]. Intravitreal DEX injections in chick retina inhibited the proliferation of progenitor MGCs through suppressing MAPK signaling [68]. Regardless of the type of retinal damage, GR agonists have been shown to protect neural retina against cytotoxic damages and to suppress reactivity of microglia [69]. Low doses of DEX significantly reversed the impairment of the cytoskeletal protein dystrophin Dp71, the water transport protein aquaporin 4 (AQP4), and the potassium channel Kir4.1 in vivo in a mouse model of partial lens surgery and ex vivo in cultured MGCs inhibiting their swelling [70]. The expression and distribution of these channels have been shown to be altered in many retinal diseases such as retinal detachment and diabetic retinopathy. Thus, low doses of DEX could be protective and beneficial in clinical practice with ultimately fewer complications.
Retinal pigment epithelium (RPE)/choroid
Retinal pigment epithelium (RPE) is a monolayer of cells connected with tight junctions forming the outer blood-retinal barrier (BRB). BRB is an important physiologic regulator of the flow of nutrients and molecules between photoreceptors and choroid, a highly vascularized layer underneath the retina. RPE dysfunction plays a critical role in many vision-threatening ocular diseases. Microarray transcriptome analysis of RPE/choroid isolated from wild-type mice treated with intravitreal DEX or TA identified unique neurotransmission signaling pathways that were differentially regulated between DEX and TA at different time points, indicating that GC’s influence is dependent on the type of steroid and treatment duration. While different steroids might be expected to exert similar actions, it has been previously established that different GR ligands induce different nuclear distribution patterns [71]. GR distribution has been shown to be significantly impacted by ligand-specific structures such as the 9-fluoro and 17-hydroxyl groups. Since DEX and TA are structurally different at these positions, this might be a partial explanation for their different actions. Meanwhile, GCs significantly regulated genes involved in circadian rhythm signaling in steroid/time-independent fashion [72]. The mammalian retina has its own circadian clock mechanism, which has been reported to be present in photoreceptors [73] and/or RPE [74], autonomously from the central circadian clock in the hypothalamus. Regulation of photoreceptor disc shedding is a rhythmic process that is known to be one of the main functions of RPE, indicating a possible vital impact of GCs on proper function and health of RPE and photoreceptors. In vitro studies on human retinal pigment epithelial cells (ARPE19) showed that DEX treatment protected glutathione pool and increased antioxidant capacity of ARPE19, opposite to TA [75], suggesting a protective role of DEX but not for TA against oxidative stress. In a rabbit model of choroidal neovascularization, intravitreal TA significantly suppressed the levels of VEGF, and inflammatory cytokines such as IL6, and monocyte chemotactic protein-1 (MCP-1) [76]. Indeed, TA has been shown to reduce vitreal levels of VEGF in patients with diabetic retinopathy, and therefore, supporting the need for combination therapy with GCs and anti-VEGF to tackle more than one target and to achieve a superior outcome in treatment.
Concluding Remarks
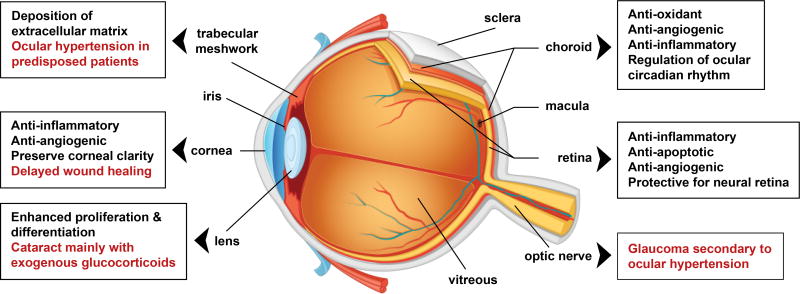
Glucocorticoids are among the most widely prescribed drugs in the ophthalmology practice (Figure 2). Given the complexity of glucocorticoid signaling and transcriptional activity, it is becoming clear that most of GCs clinical application in ophthalmology lacks knowledge of GC actions at the molecular and physiological levels. Advances in understanding the physiological role of GCs, specifically in the eye, are vital for the development of optimized targeted GC therapies of specific, and potent anti-inflammatory potential with minimal adverse effects. The current findings reinforce the need for appropriate animal models with conditional deletion of GR in specific tissues of the eye- cornea, lens, TM, retina, and RPE/choroid to dissect the mechanism of GR signaling in individual ocular layers, and then advance to understand the cross talk between them. Needless to mention that in vivo experiment in the eyes should be performed in male and female animals to account for the sexual dimorphic effects of GCs, that continued to be observed in different organs. Furthermore, using high doses of GCs in the ophthalmology clinics is a major drawback that could be contributing to the development of GC-induced complications. Therefore, it is important to study ocular glucocorticoid signaling mechanisms using dose and time-gradient of GCs to eventually characterize the lowest functional dose and optimal time point with maximum benefit and least side effects.
Figure 2.
Role of glucocorticoids in the eye. Schematic representation of major layers of the eye, demonstrated roles of glucocorticoids (black text), and reported adverse effects (red text).
Acknowledgments
This research was supported [in part] by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
References
- 1.Buttgereit F. Current issues of basic and clinical glucocorticoid research. Clin Exp Rheumatol. 2003;21(2):145–7. [PubMed] [Google Scholar]
- 2.Schacke H, Docke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96(1):23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 3.Borel JF, Feurer C, Gubler HU, Stahelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976;6(4):468–75. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- 4.Towler HM, Lightman SL, Forrester JV. Low-dose cyclosporin therapy of ocular inflammation: preliminary report of a long-term follow-up study. J Autoimmun. 1992;5(Suppl A):259–64. doi: 10.1016/0896-8411(92)90041-n. [DOI] [PubMed] [Google Scholar]
- 5.Edelman JL. Differentiating intraocular glucocorticoids. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2010;224(Suppl 1):25–30. doi: 10.1159/000315158. [DOI] [PubMed] [Google Scholar]
- 6.Busillo JM, Cidlowski JA. The five Rs of glucocorticoid action during inflammation: ready, reinforce, repress, resolve, and restore. Trends Endocrinol Metab. 2013;24(3):109–19. doi: 10.1016/j.tem.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duma D, Collins JB, Chou JW, Cidlowski JA. Sexually dimorphic actions of glucocorticoids provide a link to inflammatory diseases with gender differences in prevalence. Sci Signal. 2010;3(143):ra74. doi: 10.1126/scisignal.2001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinn M, Ramamoorthy S, Cidlowski JA. Sexually dimorphic actions of glucocorticoids: beyond chromosomes and sex hormones. Ann N Y Acad Sci. 2014;1317:1–6. doi: 10.1111/nyas.12425. [DOI] [PubMed] [Google Scholar]
- 9.Quinn MA, Cidlowski JA. Endogenous hepatic glucocorticoid receptor signaling coordinates sex-biased inflammatory gene expression. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2016;30(2):971–82. doi: 10.1096/fj.15-278309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cain DW, Cidlowski JA. Specificity and sensitivity of glucocorticoid signaling in health and disease. Best Pract Res Clin Endocrinol Metab. 2015;29(4):545–56. doi: 10.1016/j.beem.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz-Topete D, Cidlowski JA. One hormone, two actions: anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation. 2015;22(1–2):20–32. doi: 10.1159/000362724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oakley RH, Cidlowski JA. Cellular processing of the glucocorticoid receptor gene and protein: new mechanisms for generating tissue-specific actions of glucocorticoids. The Journal of biological chemistry. 2011;286(5):3177–84. doi: 10.1074/jbc.R110.179325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis-Tuffin LJ, Jewell CM, Bienstock RJ, Collins JB, Cidlowski JA. Human glucocorticoid receptor beta binds RU-486 and is transcriptionally active. Mol Cell Biol. 2007;27(6):2266–82. doi: 10.1128/MCB.01439-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly A, Bowen H, Jee YK, Mahfiche N, Soh C, Lee T, Hawrylowicz C, Lavender P. The glucocorticoid receptor beta isoform can mediate transcriptional repression by recruiting histone deacetylases. J Allergy Clin Immunol. 2008;121(1):203–208. e1. doi: 10.1016/j.jaci.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Kino T, Manoli I, Kelkar S, Wang Y, Su YA, Chrousos GP. Glucocorticoid receptor (GR) beta has intrinsic, GRalpha-independent transcriptional activity. Biochem Biophys Res Commun. 2009;381(4):671–5. doi: 10.1016/j.bbrc.2009.02.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramamoorthy S, Cidlowski JA. Corticosteroids: Mechanisms of Action in Health and Disease. Rheum Dis Clin North Am. 2016;42(1):15–31. vii. doi: 10.1016/j.rdc.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramamoorthy S, Cidlowski JA. Exploring the molecular mechanisms of glucocorticoid receptor action from sensitivity to resistance. Endocr Dev. 2013;24:41–56. doi: 10.1159/000342502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadmiel M, Cidlowski JA. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol Sci. 2013;34(9):518–30. doi: 10.1016/j.tips.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stahn C, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat Clin Pract Rheumatol. 2008;4(10):525–33. doi: 10.1038/ncprheum0898. [DOI] [PubMed] [Google Scholar]
- 20.Ayroldi E, Cannarile L, Migliorati G, Nocentini G, Delfino DV, Riccardi C. Mechanisms of the anti-inflammatory effects of glucocorticoids: genomic and nongenomic interference with MAPK signaling pathways. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26(12):4805–20. doi: 10.1096/fj.12-216382. [DOI] [PubMed] [Google Scholar]
- 21.Zhou R, Caspi RR. Ocular immune privilege. F1000 Biol Rep. 2010;2 doi: 10.3410/B2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hori J, Niederkorn JY. Immunogenicity and immune privilege of corneal allografts. Chem Immunol Allergy. 2007;92:290–9. doi: 10.1159/000099279. [DOI] [PubMed] [Google Scholar]
- 23.Qazi Y, Maddula S, Ambati BK. Mediators of ocular angiogenesis. Journal of genetics. 2009;88(4):495–515. doi: 10.1007/s12041-009-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang SX, Ma JX. Ocular neovascularization: Implication of endogenous angiogenic inhibitors and potential therapy. Progress in retinal and eye research. 2007;26(1):1–37. doi: 10.1016/j.preteyeres.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Friedlaender MH. The current and future therapy of allergic conjunctivitis. Curr Opin Ophthalmol. 1998;9(4):54–8. doi: 10.1097/00055735-199808000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Kiernan DF, Mieler WF. The use of intraocular corticosteroids. Expert Opin Pharmacother. 2009;10(15):2511–25. doi: 10.1517/14656560903160671. [DOI] [PubMed] [Google Scholar]
- 27.Miyamoto N, Iossifov D, Metge F, Behar-Cohen F. Early effects of intravitreal triamcinolone on macular edema: mechanistic implication. Ophthalmology. 2006;113(11):2048–53. doi: 10.1016/j.ophtha.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 28.Augustin AJ, Schmidt-Erfurth U. Verteporfin therapy combined with intravitreal triamcinolone in all types of choroidal neovascularization due to age-related macular degeneration. Ophthalmology. 2006;113(1):14–22. doi: 10.1016/j.ophtha.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Liggett PE, Colina J, Chaudhry NA, Tom D, Haffner G. Triple therapy of intravitreal triamcinolone, photodynamic therapy, and pegaptanib sodium for choroidal neovascularization. Am J Ophthalmol. 2006;142(6):1072–4. doi: 10.1016/j.ajo.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 30.Torriglia A, Valamanesh F, Behar-Cohen F. On the retinal toxicity of intraocular glucocorticoids. Biochem Pharmacol. 2010;80(12):1878–86. doi: 10.1016/j.bcp.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Jones R, 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Current opinion in ophthalmology. 2006;17(2):163–7. doi: 10.1097/01.icu.0000193079.55240.18. [DOI] [PubMed] [Google Scholar]
- 32.Ramu J, Chatziralli I, Yang Y, Menon G, Bailey C, Eckstein M, Hykin P, Sivaprasad S. Health-related quality of life, visual function and treatment satisfaction following intravitreal dexamethasone implant for diabetic macular edema. Patient Prefer Adherence. 2017;11:579–586. doi: 10.2147/PPA.S132859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirabelli P, Peebo BB, Xeroudaki M, Koulikovska M, Lagali N. Early effects of dexamethasone and anti-VEGF therapy in an inflammatory corneal neovascularization model. Exp Eye Res. 2014;125:118–27. doi: 10.1016/j.exer.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Saud EE, Moraes HV, Jr, Marculino LG, Gomes JA, Allodi S, Miguel NC. Clinical and histopathological outcomes of subconjunctival triamcinolone injection for the treatment of acute ocular alkali burn in rabbits. Cornea. 2012;31(2):181–7. doi: 10.1097/ICO.0b013e318221ce99. [DOI] [PubMed] [Google Scholar]
- 35.Ryu JS, Ko JH, Kim MK, Wee WR, Oh JY. Prednisolone induces apoptosis in corneal epithelial cells through the intrinsic pathway. Scientific reports. 2017;7(1):4135. doi: 10.1038/s41598-017-04509-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kadmiel M, Janoshazi A, Xu X, Cidlowski JA. Glucocorticoid action in human corneal epithelial cells establishes roles for corticosteroids in wound healing and barrier function of the eye. Experimental eye research. 2016;152:10–33. doi: 10.1016/j.exer.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bian F, Pelegrino FS, Henriksson JT, Pflugfelder SC, Volpe EA, Li DQ, de Paiva CS. Differential Effects of Dexamethasone and Doxycycline on Inflammation and MMP Production in Murine Alkali-Burned Corneas Associated with Dry Eye. Ocul Surf. 2016;14(2):242–54. doi: 10.1016/j.jtos.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bian F, Wang C, Tukler-Henriksson J, Pflugfelder SC, Camodeca C, Nuti E, Rossello A, Li DQ, de Paiva CS. MMP-8 Is Critical for Dexamethasone Therapy in Alkali-Burned Corneas Under Dry Eye Conditions. Journal of cellular physiology. 2016;231(11):2506–16. doi: 10.1002/jcp.25364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Onyimba CU, Vijapurapu N, Curnow SJ, Khosla P, Stewart PM, Murray PI, Walker EA, Rauz S. Characterisation of the prereceptor regulation of glucocorticoids in the anterior segment of the rabbit eye. J Endocrinol. 2006;190(2):483–93. doi: 10.1677/joe.1.06840. [DOI] [PubMed] [Google Scholar]
- 40.Susarla R, Liu L, Walker EA, Bujalska IJ, Alsalem J, Williams GP, Sreekantam S, Taylor AE, Tallouzi M, Southworth HS, Murray PI, Wallace GR, Rauz S. Cortisol biosynthesis in the human ocular surface innate immune response. PloS one. 2014;9(4):e94913. doi: 10.1371/journal.pone.0094913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao J, Yang W, Liu Y, Sun YX, Jiang Q. Dexamethasone inhibits TGF-beta2-induced migration of human lens epithelial cells: implications for posterior capsule opacification prevention. Mol Med Rep. 2012;5(6):1509–13. doi: 10.3892/mmr.2012.827. [DOI] [PubMed] [Google Scholar]
- 42.Zhou D, Zhang Y, Wang L, Sun Y, Liu P. Identification of genes and transcription factors associated with glucocorticoid response in lens epithelial cells. Mol Med Rep. 2015;11(6):4073–8. doi: 10.3892/mmr.2015.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janciauskiene S, Westin K, Grip O, Krakau T. Detection of Alzheimer peptides and chemokines in the aqueous humor. European journal of ophthalmology. 2011;21(1):104–11. doi: 10.5301/ejo.2010.2108. [DOI] [PubMed] [Google Scholar]
- 44.Wang C, Dawes LJ, Liu Y, Wen L, Lovicu FJ, McAvoy JW. Dexamethasone influences FGF-induced responses in lens epithelial explants and promotes the posterior capsule coverage that is a feature of glucocorticoid-induced cataract. Experimental eye research. 2013;111:79–87. doi: 10.1016/j.exer.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamberts SW. Glucocorticoid receptors and Cushing's disease. Mol Cell Endocrinol. 2002;197(1–2):69–72. doi: 10.1016/s0303-7207(02)00280-0. [DOI] [PubMed] [Google Scholar]
- 46.Gabelt BT, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Progress in retinal and eye research. 2005;24(5):612–37. doi: 10.1016/j.preteyeres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Kersey JP, Broadway DC. Corticosteroid-induced glaucoma: a review of the literature. Eye. 2006;20(4):407–16. doi: 10.1038/sj.eye.6701895. [DOI] [PubMed] [Google Scholar]
- 48.Fini ME, Schwartz SG, Gao X, Jeong S, Patel N, Itakura T, Price MO, Price FW, Jr, Varma R, Stamer WD. Steroid-induced ocular hypertension/glaucoma: Focus on pharmacogenomics and implications for precision medicine. Progress in retinal and eye research. 2017;56:58–83. doi: 10.1016/j.preteyeres.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bollinger KE, Crabb JS, Yuan X, Putliwala T, Clark AF, Crabb JW. Proteomic similarities in steroid responsiveness in normal and glaucomatous trabecular meshwork cells. Molecular vision. 2012;18:2001–11. [PMC free article] [PubMed] [Google Scholar]
- 50.Clark R, Nosie A, Walker T, Faralli JA, Filla MS, Barrett-Wilt G, Peters DM. Comparative genomic and proteomic analysis of cytoskeletal changes in dexamethasone-treated trabecular meshwork cells. Molecular & cellular proteomics : MCP. 2013;12(1):194–206. doi: 10.1074/mcp.M112.019745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bermudez JY, Webber HC, Brown B, Braun TA, Clark AF, Mao W. A Comparison of Gene Expression Profiles between Glucocorticoid Responder and Non-Responder Bovine Trabecular Meshwork Cells Using RNA Sequencing. PloS one. 2017;12(1):e0169671. doi: 10.1371/journal.pone.0169671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuan Y, Call MK, Yuan Y, Zhang Y, Fischesser K, Liu CY, Kao WW. Dexamethasone induces cross-linked actin networks in trabecular meshwork cells through noncanonical wnt signaling. Investigative ophthalmology & visual science. 2013;54(10):6502–9. doi: 10.1167/iovs.13-12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raghunathan VK, Morgan JT, Park SA, Weber D, Phinney BS, Murphy CJ, Russell P. Dexamethasone Stiffens Trabecular Meshwork, Trabecular Meshwork Cells, and Matrix. Investigative ophthalmology & visual science. 2015;56(8):4447–59. doi: 10.1167/iovs.15-16739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bermudez JY, Montecchi-Palmer M, Mao W, Clark AF. Cross-linked actin networks (CLANs) in glaucoma. Experimental eye research. 2017;159:16–22. doi: 10.1016/j.exer.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stamer WD, Hoffman EA, Kurali E, Krauss AH. Unique response profile of trabecular meshwork cells to the novel selective glucocorticoid receptor agonist, GW870086X. Investigative ophthalmology & visual science. 2013;54(3):2100–7. doi: 10.1167/iovs.12-11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dismuke WM, Klingeborn M, Stamer WD. Mechanism of Fibronectin Binding to Human Trabecular Meshwork Exosomes and Its Modulation by Dexamethasone. PloS one. 2016;11(10):e0165326. doi: 10.1371/journal.pone.0165326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zode GS, Sharma AB, Lin X, Searby CC, Bugge K, Kim GH, Clark AF, Sheffield VC. Ocular-specific ER stress reduction rescues glaucoma in murine glucocorticoid-induced glaucoma. The Journal of clinical investigation. 2014;124(5):1956–65. doi: 10.1172/JCI69774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel GC, Phan TN, Maddineni P, Kasetti RB, Millar JC, Clark AF, Zode GS. Dexamethasone-Induced Ocular Hypertension in Mice: Effects of Myocilin and Route of Administration. The American journal of pathology. 2017;187(4):713–723. doi: 10.1016/j.ajpath.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jain A, Wordinger RJ, Yorio T, Clark AF. Role of the alternatively spliced glucocorticoid receptor isoform GRbeta in steroid responsiveness and glaucoma. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2014;30(2–3):121–7. doi: 10.1089/jop.2013.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gu R, Lei B, Shu Q, Li G, Xu G. Glucocorticoid-induced leucine zipper overexpression inhibits lipopolysaccharide-induced retinal inflammation in rats. Experimental eye research. 2017 doi: 10.1016/j.exer.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 61.Gu R, Lei B, Jiang C, Xu G. Glucocorticoid-Induced Leucine Zipper Suppresses ICAM-1 and MCP-1 Expression by Dephosphorylation of NF-kappaB p65 in Retinal Endothelial Cells. Investigative ophthalmology & visual science. 2017;58(1):631–641. doi: 10.1167/iovs.16-20933. [DOI] [PubMed] [Google Scholar]
- 62.Zhang X, Lai D, Bao S, Hambly BD, Gillies MC. Triamcinolone acetonide inhibits p38MAPK activation and neuronal apoptosis in early diabetic retinopathy. Curr Mol Med. 2013;13(6):946–58. doi: 10.2174/1566524011313060007. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y, Zhang Z, Zhang L, Li X, Lu R, Xu P, Zhang X, Dai M, Dai X, Qu J, Lu F, Chi Z. S100A8 promotes migration and infiltration of inflammatory cells in acute anterior uveitis. Scientific reports. 2016;6:36140. doi: 10.1038/srep36140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cubilla MA, Bermudez V, Marquioni Ramella MD, Bachor TP, Suburo AM. Mifepristone, a blocker of glucocorticoid receptors, promotes photoreceptor death. Invest Ophthalmol Vis Sci. 2013;54(1):313–22. doi: 10.1167/iovs.12-10014. [DOI] [PubMed] [Google Scholar]
- 65.Stewart EA, Saker S, Amoaku WM. Dexamethasone reverses the effects of high glucose on human retinal endothelial cell permeability and proliferation in vitro. Experimental eye research. 2016;151:75–81. doi: 10.1016/j.exer.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 66.Adachi T, Teramachi M, Yasuda H, Kamiya T, Hara H. Contribution of p38 MAPK, NF-kappaB and glucocorticoid signaling pathways to ER stress-induced increase in retinal endothelial permeability. Archives of biochemistry and biophysics. 2012;520(1):30–5. doi: 10.1016/j.abb.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 67.El Zaoui I, Behar-Cohen F, Torriglia A. Glucocorticoids exert direct toxicity on microvasculature: analysis of cell death mechanisms. Toxicol Sci. 2015;143(2):441–53. doi: 10.1093/toxsci/kfu243. [DOI] [PubMed] [Google Scholar]
- 68.Gallina D, Zelinka C, Fischer AJ. Glucocorticoid receptors in the retina, Muller glia and the formation of Muller glia-derived progenitors. Development. 2014;141(17):3340–51. doi: 10.1242/dev.109835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gallina D, Zelinka CP, Cebulla CM, Fischer AJ. Activation of glucocorticoid receptors in Muller glia is protective to retinal neurons and suppresses microglial reactivity. Exp Neurol. 2015;273:114–25. doi: 10.1016/j.expneurol.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Siqueiros-Marquez L, Benard R, Vacca O, Charles-Messance H, Bolanos-Jimenez R, Guilloneau X, Sennlaub F, Montanez C, Sahel JA, Rendon A, Tadayoni R, Giocanti-Auregan A. Protection of Glial Muller Cells by Dexamethasone in a Mouse Model of Surgically Induced Blood-Retinal Barrier Breakdown. Investigative ophthalmology & visual science. 2017;58(2):876–886. doi: 10.1167/iovs.16-20617. [DOI] [PubMed] [Google Scholar]
- 71.Schaaf MJ, Lewis-Tuffin LJ, Cidlowski JA. Ligand-selective targeting of the glucocorticoid receptor to nuclear subdomains is associated with decreased receptor mobility. Mol Endocrinol. 2005;19(6):1501–15. doi: 10.1210/me.2005-0050. [DOI] [PubMed] [Google Scholar]
- 72.Smit-McBride Z, Moisseiev E, Modjtahedi SP, Telander DG, Hjelmeland LM, Morse LS. Comparison of In Vivo Gene Expression Profiling of RPE/Choroid following Intravitreal Injection of Dexamethasone and Triamcinolone Acetonide. J Ophthalmol. 2016;2016:9856736. doi: 10.1155/2016/9856736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tosini G, Davidson AJ, Fukuhara C, Kasamatsu M, Castanon-Cervantes O. Localization of a circadian clock in mammalian photoreceptors. FASEB J. 2007;21(14):3866–71. doi: 10.1096/fj.07-8371com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peirson SN, Bovee-Geurts PH, Lupi D, Jeffery G, DeGrip WJ, Foster RG. Expression of the candidate circadian photopigment melanopsin (Opn4) in the mouse retinal pigment epithelium. Brain Res Mol Brain Res. 2004;123(1–2):132–5. doi: 10.1016/j.molbrainres.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 75.Raffaele N, Marchese A, Ghigo D. Compared antioxidant activity among corticosteroids on cultured retinal pigment epithelial cells. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2016;254(12):2411–2416. doi: 10.1007/s00417-016-3519-3. [DOI] [PubMed] [Google Scholar]
- 76.Arimura S, Takamura Y, Miyake S, Gozawa M, Iwasaki K, Tomomatsu T, Matsumura T, Inatani M. The effect of triamcinolone acetonide or bevacizumab on the levels of proinflammatory cytokines after retinal laser photocoagulation in pigmented rabbits. Experimental eye research. 2016;149:1–7. doi: 10.1016/j.exer.2016.06.004. [DOI] [PubMed] [Google Scholar]