Table 2.
Activities and stereoselectivities of biocatalysts for the reaction of styrene with ethyl diazoacetate.a
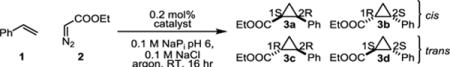
| |||||
|---|---|---|---|---|---|
| catalyst | yield [%] | TTNb | dr (cis:trans) | ee cis [%]c | ee trans [%]d |
| heme | 16 | 80 | 13:87 | 0 | −3 |
| Ir(Me)-DPIX | 40 | 200 | 29:71 | −1 | −4 |
| BM3h-T268A/ heme | 68 | 339 | 1:99 | −11 | −97 |
| WIVS-FM*-T268A/C400G/Ir(Me)-DPIX | 48 | 240 | 29:71 | 0 | 0 |
reaction conditions: 7.5 mM olefin, 10 mM EDA, 15 μM catalyst, in 0.1 M NaPI pH 6.0 100 mM NaCl buffer, 5-% DMF as cosolvent (reactions with heme and heme enzymes also contain 10 mM dithionite). TTN and stereoselectivities determined by chiral GC analysis.
TTN = total turnover number.
(1S, 2R) – (1R, 2S).
(1R, 2R) – (1S, 2S).
