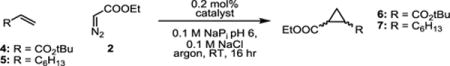
Table 3.
Activities and diastereoselectivities of biocatalysts for the reaction of electron-deficient and aliphatic olefins with ethyl diazoacetate.a
| ||||
|---|---|---|---|---|
| catalyst | substrate | yield [%] | TTNb | drc,d (cis:trans) |
| heme | 4 | 6 | 29 | 31:69 |
| BM3h-T268A/heme | 4 | 7 | 35 | 43:57 |
| Ir(Me)-DPIX | 4 | 8 | 40 | 14:86 |
| WIVS-FM*-T268A/C400G/Ir(Me)-DPIX | 4 | 29 | 147 | 13:87 |
| heme | 5 | n.d.e | n.d.e | n.d.e |
| BM3h-T268A/heme | 5 | n.d.e | n.d.e | n.d.e |
| Ir(Me)-DPIX | 5 | 5 | 23 | 30:70 |
| WIVS-FM*-T268A/C400G/Ir(Me)-DPIX | 5 | 11 | 55 | 26:74 |
| WIVS-FM*-F87W/T268A/C400G/Ir(Me)-DPIX | 5 | 19 | 95 | 26:74 |
reaction conditions: 100 mM olefin, 10 mM EDA, 20 μM catalyst, in 0.1 M NaPI pH 6.0 100 mM NaCl buffer, 5% DMF as cosolvent (reactions with heme and heme enzymes also contain 10 mM dithionite). TTN and diastereoselectivities determined by chiral GC analysis. Enantiomers could not be resolved by chiral GC.
TTN = total turnover number.
Diastereomer identity determined by comparison to authentic standards.
Enantioselectivities are reported in table S4 and S5.
n.d. = not detected.
