Abstract
The initiation of hemodialysis is associated with an accelerated decline of cognitive function and an increased incidence of cerebrovascular accidents and white matter lesions. Investigators have hypothesized that the repetitive circulatory stress of hemodialysis induces ischemic cerebral injury, but the mechanism is unclear. We studied the acute effect of conventional hemodialysis on cerebral blood flow (CBF), measured by [15O]H2O positron emission tomography–computed tomography (PET-CT). During a single hemodialysis session, three [15O]H2O PET-CT scans were performed: before, early after the start of, and at the end of hemodialysis. We used linear mixed models to study global and regional CBF change during hemodialysis. Twelve patients aged ≥65 years (five women, seven men), with a median dialysis vintage of 46 months, completed the study. Mean (±SD) arterial BP declined from 101±11 mm Hg before hemodialysis to 93±17 mm Hg at the end of hemodialysis. From before the start to the end of hemodialysis, global CBF declined significantly by 10%±15%, from a mean of 34.5 to 30.5 ml/100g per minute (difference, −4.1 ml/100 g per minute; 95% confidence interval, −7.3 to −0.9 ml/100 g per minute; P=0.03). CBF decline (20%) was symptomatic in one patient. Regional CBF declined in all volumes of interest, including the frontal, parietal, temporal, and occipital lobes; cerebellum; and thalamus. Higher tympanic temperature, ultrafiltration volume, ultrafiltration rate, and pH significantly associated with lower CBF. Thus, conventional hemodialysis induces a significant reduction in global and regional CBF in elderly patients. Repetitive intradialytic decreases in CBF may be one mechanism by which hemodialysis induces cerebral ischemic injury.
Keywords: geriatric nephrology, brain perfusion, chronic renal failure, [15O]H2O PET, executive function
More than 2 million individuals with ESRD worldwide receive RRT, of which hemodialysis (HD) is the most frequently used modality.1,2 Especially in elderly patients receiving HD cognitive impairment is highly common, with a prevalence up to 60%.3–5 Decline of cognitive function, especially of executive function, is already present in patients with mild-to-moderate CKD and the transition to dialysis is associated with a significant loss of executive function.6–10
There is increasing evidence that the HD procedure itself might contribute to brain injury. First, it was reported that stroke incidence rose in the first month of HD in elderly patients and remained elevated afterward compared with the period before initiation of HD.11 Second, a longer HD vintage is associated with reduced white matter integrity on magnetic resonance imaging (MRI).12–14 Finally, lowering the dialysate temperature resulted in an improvement in intradialytic hemodynamic stability and strongly attenuated the progression of white matter lesions during the first year of HD, providing indirect evidence that the HD procedure contributes to cerebral ischemia.15 At present, the mechanism by which HD could contribute to brain damage is unknown. For the heart, it was shown that HD induces a fall in myocardial blood flow resulting in subclinical myocardial ischemia.16–19 Likewise, we hypothesized that a repetitive HD-induced cerebral blood flow (CBF) decline may lead to (cumulative) ischemic brain lesions. These lesions may contribute to the accelerated cognitive decline after the initiation of HD. To our knowledge, no study has yet evaluated the acute effect of HD on CBF using quantitative CBF measurements. We aimed to study the effect of HD on CBF early and late during the dialysis procedure using [15O]H2O positron emission tomography–computed tomography (PET-CT) scans, which are considered the gold standard for CBF measurement.20–22 The primary objective was to evaluate the effect of HD on global and regional CBF. The secondary objective was to explore associations of HD treatment–related factors with CBF.
Results
Enrolment and Patient Characteristics
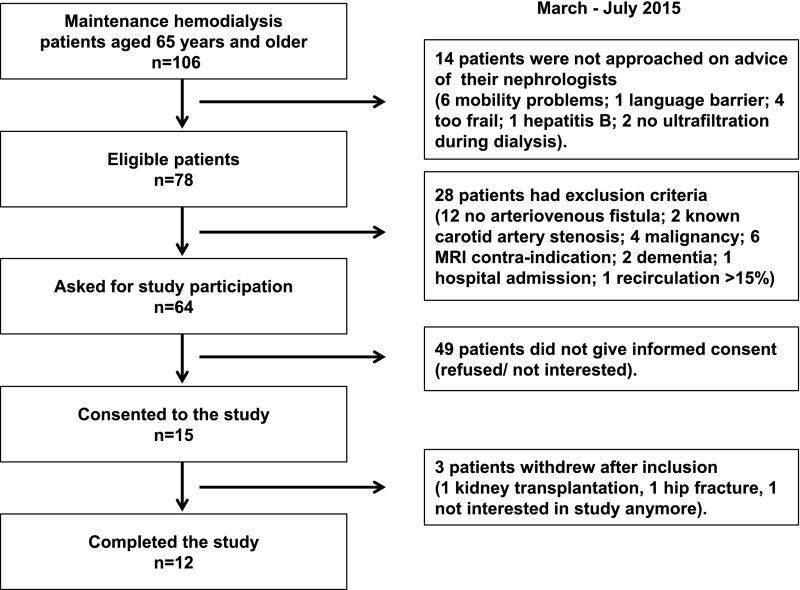
Of 78 eligible patients aged ≥65 years, 64 patients were asked to participate, and 15 patients gave written informed consent (Figure 1). None of the patients had to be excluded because of a significant carotid artery stenosis. Three patients withdrew from the study, because of a kidney transplantation, hip fracture, and withdrawal of consent, respectively. Twelve patients completed the study, of whom the characteristics are summarized in Table 1.
Figure 1.
Flow chart demonstrating the phases of the study from screening to inclusion and completion of the study.
Table 1.
Patient characteristics
| Characteristic | Total, n=12 |
|---|---|
| Age | 75.4±5.2 |
| Men | 7 (58%) |
| BMI, kg/m2 | 26.6±3.5 |
| Primary kidney disease: | |
| GN | 4 (33%) |
| Diabetes | 1 (8%) |
| Vascular | 3 (25%) |
| Other diagnosis | 3 (25%) |
| Unknown | 1 (8%) |
| Dialysis vintage, mo | 46 (range 11–319) |
| Dialysis treatment time, h/wk | 12 (range 8–15) |
| Kt/V, per wk | 3.91±0.73 |
| % IDH-complicated HD sessions 30 d before study sessiona | |
| Never | 8 (67%) |
| In 10%–20% of HD sessions | 2 (17%) |
| In 30%–40% of HD sessions | 2 (17%) |
| Comorbidities | |
| Diabetes | 3 (25%) |
| Hypertension | 11 (73%) |
| Myocardial infarction | 2 (17%) |
| Heart failure | 1 (8%) |
| Peripheral artery disease | 1 (8%) |
| COPD | 1 (8%) |
| Depression | 1 (8%) |
| Medication | |
| CCB | 4 (33%) |
| Nitrate | 3 (25%) |
| ACE inhibitor | 1 (8%) |
| Angiotensin receptor blocker | 1 (8%) |
| B-blocker | 9 (75%) |
| Neuropsychologic assessment | |
| MMSEb | 28 (range 25–29) |
| RAVLT, delayed recallc | 6.8±3.4 |
| Digit span forwardc | 5.1±0.8 |
| Digit span backwardc | 3.8±1.1 |
| TMT-A, secc | 71.3±28.6 |
| TMT-B, secc | 200±94 |
| TMT B/A ratiod | 2.59±0.90 |
| Letter fluencyc | 24.6±12.1 |
| Clock drawing scorec | 14 (range 9–14) |
| HADS depression scoree | 6.0±3.9 |
| HADS anxiety score | 3.9±3.6 |
| MRI brain | |
| GCA score | |
| 0 – no atrophy | 1 (8%) |
| 1 – mild atrophy | 8 (67%) |
| 2 – moderate atrophy | 3 (25) |
| 3 – severe atrophy | 0 |
| Fazekas score of WML | |
| 0 – no WML | 1 (8%) |
| 1 – multiple punctate lesions | 4 (33%) |
| 2 – beginning confluent lesions | 5 (42%) |
| 3 – large confluent WML | 2 (17%) |
| Microbleeds | 7 (58%) |
Data are presented as mean±SD or median (range), or percentages (%). BMI, body mass index; IDH, intradialytic hypotension; COPD, chronic obstructive pulmonary disease; CCB, calcium channel blocker; ACE, angiotensin-converting enzyme; MMSE, Mini Mental State Examination; RAVLT, Rey Auditory Verbal Learning Test; TMT, trail making test; HADS, hospital anxiety depression scale; GCA, global cortical atrophy; WML, white matter lesions.
IDH was defined as an SBP drop <100 mm Hg, any IDH-related intervention during HD, or IDH symptoms including dizziness or loss of consciousness.
An MMSE score ≥24 indicates normal cognition.
The number of patients that were impaired according the age-, sex-, and education-adjusted norm scores were: 0 (RAVLT), 2 (total digit span score, only age-adjusted), 4 (TMT-A, TMT-B), 2 (letter fluency), and 1 (clock drawing).
Three patients had a TMT B/A ratio >3.0 indicating executive function impairment.
A score >7 on the HADS depression (n=3) or anxiety (n=1) indicates the presence of symptoms of depression or anxiety, respectively.
HD Study Session Characteristics
During a single HD session three [15O]H2O PET-CT scans were performed: Before (T1), shortly after the start of HD (T2), and at the end of HD (T3). The mean time interval between T1 and T2 was 39 minutes (range, 28–61 minutes). The second and third scans were performed at a mean of 21 minutes (range, 13–29 minutes) and 209 minutes (range, 168–223 minutes) after the start of HD, respectively. Intradialytic changes in vital and laboratory parameters are shown in Table 2. Mean ultrafiltration (UF) volume was 1934±781 ml, UF rate 6.7±2.5 ml/h per kilogram, and weight change −1.6±0.7 kg.
Table 2.
Intradialytic changes in vital and laboratory values
| Characteristic | Before Start HD T1 | After Start HD T2 | At the End of HD T3 | Dialysis Treatment Effect | |
|---|---|---|---|---|---|
| T1 versus T3 | T2 versus T3 | ||||
| SBP, mm Hg | 152±22 | 157±26 | 140±30 | −9 (−27 to 10) | −15 (−36 to 5) |
| DBP, mm Hg | 75±8 | 78±13 | 70±12 | −5 (−14 to 4) | −7 (−17 to 3) |
| MAP, mm Hg | 101±11 | 105±15 | 93±17 | −6 (−15 to 3) | −10 (−19 to −0.1)a |
| Heart rate, bpm | 69±9 | 68±10 | 72±9 | 4 (−3 to 11) | 5 (1 to 12) |
| Tympanic temperature | 36.3±0.5 | 36.2±0.5 | 35.9±0.6 | −0.3 (−0.8 to 0.3) | 0.1 (−0.3 to 0.6) |
| Hemoglobin, mmol/L | 6.7±0.8 | 6.4±0.9 | 7.1±0.9 | 0.4 (0.1 to 0.7)a | 0.7 (−0.4 to 1.0)b |
| Hematocrit, v/v | 0.33±0.04 | 0.31±0.04 | 0.34±0.04 | 0.02 (0.01 to 0.03)a | 0.03 (0.02 to 0.05)b |
| Glucose, mmol/L | 6.4±1.5 | 5.7±1.1 | 7.7±1.1 | 1.3 (−0.5 to 3.2) | 2.0 (0.6 to 3.4)c |
| pO2, kPa | 12.2±2.1 | 11.5±1.8 | 12.5±2.6 | 0.4 (−1.4 to 2.2) | 1.0 (−0.6 to 2.6) |
| pCO2, kPa | 5.0±0.5 | 5.2±0.5 | 5.1±0.5 | 0.1 (−0.1 to 0.3) | −0.02 (−0.4 to 0.3) |
| pH | 7.38±0.04 | 7.40±0.03 | 7.48±0.04 | 0.10 (0.07 to 0.13)b | 0.08 (0.05 to 0.11)b |
| Creatinine, μmol/L | 798±190 | 713±176 | 313±95 | −485 (−588 to −382)b | −400 (−488 to −312)b |
| Urea, mmol/L | 24.0±6.6 | 21.7±6.5 | 8.3±2.3 | −15.7 (−20.4 to −11.1)b | −13.5 (−18.1 to −8.8)b |
| Sodium, mmol/L | 139±2 | 139±2 | 141±2 | 1.8 (−0.03 to 3.7) | 1.3 (−0.6 to 3.2) |
| Potassium, mmol/L | 5.1±0.9 | 4.7±1.0 | 3.4±0.4 | −1.6 (−2.4 to −0.9)b | −1.3 (−2.0 to −0.6)c |
| Bicarbonate, mmol/L | 22±2 | 23±2 | 28±2 | 6.5 (4.8 to 8.3)b | 4.9 (3.5 to 6.4)b |
| i-Calcium, mmol/L | 1.18±0.05 | 1.20±0.06 | 1.23±0.08 | 0.05 (0.01 to 0.09)a | 0.03 (0.01 to 0.06)a |
| Lactate, mmol/L | 1.02±0.36 | 0.69±0.21 | 1.37±0.53 | 0.35 (0.03 to 0.70)a | 0.68 (0.33 to 1.02)c |
| CRP, mg/L | 7.9±6.3 | 7.5±6.1 | 8.8±8.0 | 0.9 (−1.1 to 2.9) | 1.4 (−0.5 to 3.2) |
| PTX 3, ng/ml | 1.89±0.83 | 2.08±1.36 | 3.62±1.72 | 1.73 (−2.77 to −0.69)c | 1.54 (0.98 to 2.09)b |
| MPO | 1.00±0.19 | 1.76±0.69 | 1.38±0.33 | 0.38 (0.08 to 0.68)a | −0.39 (−0.86 to 0.09) |
| vWF, % | 158±43 | 141±45 | 160±49 | 0.1 (−22 to 23) | 15 (−6 to 37) |
Data are presented as unadjusted mean±SD. Dialysis treatment effects are presented as mean differences (95% CI) obtained from repeated measurements ANOVA models. DBP, diastolic BP; i-Calcium, ionized calcium; CRP, C-reactive protein; PTX 3, pentraxin 3; MPO, myeloperoxidase; vWF, von Willebrand factor.
P<0.05 adjusted for multiple comparisons by Bonferroni.
P<0.001 adjusted for multiple comparisons by Bonferroni.
P<0.01 adjusted for multiple comparisons by Bonferroni.
The Effect of HD on Systemic BP and CBF
Mean arterial pressure (MAP) initially increased from 101±11 (T1) to 105±15 (T2) and then decreased significantly to 93±17 mm Hg at the end of HD (T3). The lowest individual nadir in systolic BP (SBP) during the HD study session was 105 mm Hg. The change in SBP and MAP between the start of HD and the nadir during HD ranged from −46 to +3 mm Hg, and from −23.3 to +9.7 mm Hg, respectively (Supplemental Table 1).
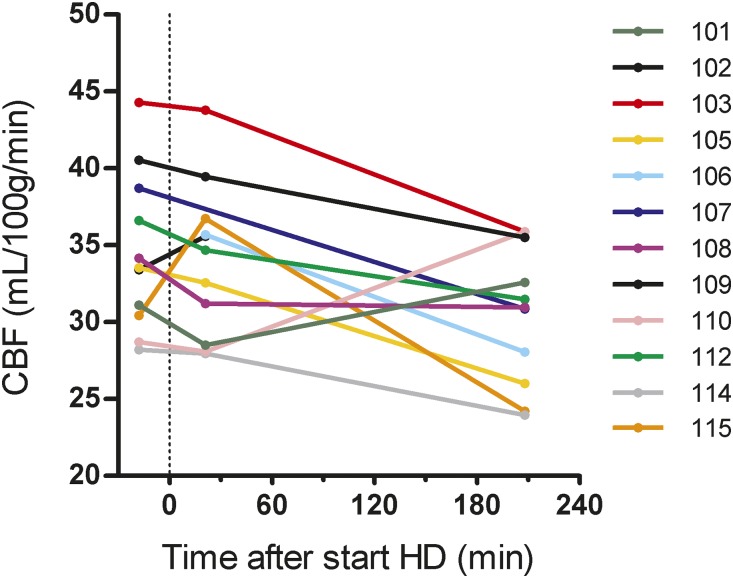
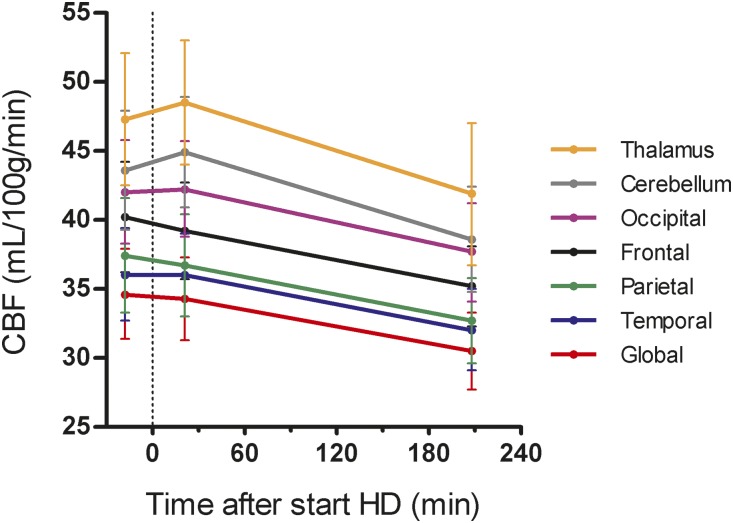
Global crude CBF levels of the individual patients are shown in Figure 2. On average, global CBF declined from a baseline of 34.5 (31.4–37.9) ml/100 g per minute to 30.5 (27.7–33.3) ml/100 g per minute at the end of HD in the linear mixed models (LMM) analysis (difference, −4.1 ml/100 g per minute; 95% confidence interval [95% CI], −7.3 to −0.9; P=0.03) (Table 3). Regionally, CBF declined in all volumes of interest (VOIs) (Figure 3, Table 3).
Figure 2.
Individual crude CBF trajectories during HD. Scan 1 was performed at a mean of 18 minutes (range, 15–31 minutes) before the start of HD. HD is regarded as baseline (t=0). Scan 2 and scan 3 were performed at a mean of 21 minutes (range, 13–29 minutes) and 209 minutes (range, 168–223 minutes) after the start of HD, respectively. Each line represents one patient. In three patients, CBF trajectories are incomplete because one scan was missing: identity 102: T3; identity 106: T1; and identity 107: T2.
Table 3.
Intradialytic changes in CBF (ml/100 g per minute)
| Brain Region | Before Start HD T1 | After Start HDa T2 | At the End of HDa T3 | Dialysis Treatment Effectb | |
|---|---|---|---|---|---|
| T1 versus T3 | T2 versus T3 | ||||
| Global | 34.5±5.1 | 34.0±5.0 | 30.5±4.4 | −4.1 (−7.3 to −0.9)c | −3.8 (−7.2 to −0.5)c |
| Regional | |||||
| Frontal lobe | 40.2±6.9 | 38.9±5.6 | 35.0±4.7 | −5.1 (−9.5 to −0.6)c | −4.1 (−7.8 to −0.3)c |
| Parietal lobe | 37.4±7.0 | 36.3±6.2 | 32.6±5.1 | −4.7 (−8.7 to −0.8)c | −4.0 (−7.4 to −0.6)c |
| Temporal lobe | 35.8±5.1 | 35.7±5.6 | 31.8±4.7 | −4.0 (−7.4 to −0.6)c | −4.0 (−6.9 to −1.0)d |
| Occipital lobe | 41.9±5.1 | 41.6±5.1 | 37.7±5.6 | −4.4 (−8.4 to −0.3)c | −4.5 (−8.1 to −1.0)d |
| Cerebellum | 43.3±6.8 | 44.8±7.4 | 38.4±6.2 | −5.0 (−9.2 to −0.8)c | −6.3 (−10.0 to −2.6)e |
| Thalamus | 47.3±7.2 | 48.1±8.4 | 41.7±8.3 | −5.5 (−11.1 to 0.2) | −6.6 (−11.5 to −1.7)d |
Data are presented as unadjusted mean±SD.
Scan 2 and 3 were performed at mean 21 and 209 minutes after start of HD, respectively.
Dialysis treatment effects are obtained from linear mixed effects models and presented as mean difference (95% CI).
P<0.05.
P<0.01.
P<0.001.
Figure 3.
Global and regional CBF declined during HD. The CBF trajectories are shown with 95% CI's (vertical lines), and were calculated from least squares means according to LMM. Scan 1 was performed at a mean of 18 minutes (range, 15–31 minutes) before the start of HD. HD is regarded as baseline (t=0). Scan 2 and scan 3 were performed at a mean of 21 minutes (range, 13–29 minutes) and 209 minutes (range, 168–223 minutes) after the start of HD, respectively.
The relative change in crude CBF between T1 and T3 could be calculated for ten patients. Using descriptive statistics, the average (±SD) change in CBF was −10%±15% for global, −11%±17% for frontal, −11%±16% for parietal, −10%±14% for temporal, −9%±13% for occipital, −10%±13% for cerebellum, and −10%±16% for thalamus perfusion.
Associations of HD Treatment–Related Factors with CBF
To investigate the secondary objective, we explored a priori selected HD treatment–related factors that might potentially explain an intradialytic CBF change, using LMM. A higher UF volume, a higher tympanic temperature, and a lower pCO2 were associated with a lower CBF in almost all VOIs (Table 4). A higher UF rate was associated with lower frontal and temporal CBF (estimated effect, −1.2 ml/100 g per minute; 95% CI, −2.1 to −0.1; P=0.03 on frontal CBF; and −1.2 ml/100 g per minute; 95% CI, −2.0 to −0.3; P=0.02 on temporal CBF). A significant interaction of pH with scan-order was present for the association between pH and CBF in almost all VOIs. Higher pH was significantly associated with a lower regional CBF at T2 as compared with T1, but not at T3, except for frontal CBF (estimated interaction effect pH*T3, −27.4 ml/100g per minute; 95% CI, −44.9 to −3.8; P<0.001). Hematocrit was only associated with CBF in one VOI. When pH, UF volume, or tympanic temperature were added to the model, the effect of scan-order became nonsignificant. The analysis of MAP and CBF was limited by insufficient power due to considerable patient variation in MAP.
Table 4.
Associations of a priori selected HD treatment–related factors with CBF
| Variable | pCO2 (kPa) |
pH (per 0.1 change) | Temperature (°C) |
UF Volume (L) |
|
|---|---|---|---|---|---|
| Estimated effect on CBF (ml/100 g per min) | Estimated effect on CBF (ml/100 g per min) | Estimated effect on CBF (ml/100 g per min) | Estimated effect on CBF (ml/100 g per min) | ||
| pH | pH*T2 | ||||
| Interaction with scan-ordera | No | Yes | Yes | No | No |
| Region | |||||
| Global | 2.2 (−1.1 to 5.4) | 1.9 (−4.5 to 8.1) | −5.7 (−10.7 to −5.7) | 0.04 (−2.9 to 3.1) | −3.7 (−6.0 to −1.3)b |
| Regional | |||||
| Frontal lobe | 3.5 (1.1 to 5.7)b | 7.0 (1.7 to 11.0)b | −9.7 (−12.7 to −5.3)c | −2.1 (−3.3 to −0.7)b | −4.6 (−6.9 to -2.1)b |
| Parietal lobe | 4.2 (2.0 to 6.2)c | 2.7 (−2.0 to 7.0) | −7.1 (−10.0 to −3.8)c | −2.5 (−3.9 to −1.0)b | −4.7 (−7.5 to −1.6)b |
| Temporal lobe | 3.1 (0.9 to 5.3)b | 1.5 (−2.8 to 5.7) | −5.8 (−8.8 to −2.8)c | −1.7 (−2.8 to −0.6)b | −4.6 (−6.9 to −2.2)b |
| Occipital lobe | 4.4 (0.9 to 7.7)d | −1.6 (−8.4 to 4.5) | −6.6 (−11.6 to −1.6)d | −3.0 (−4.9 to −0.9)b | −5.0 (−7.8 to −1.9)b |
| Cerebellum | 3.9 (0.5 to 7.2)d | 2.5 (−3.9 to 8.6) | −8.4 (−13.4 to −3.7)b | −2.5 (−3.6 to −1.3)c | −4.7 (−8.2 to −1.0)d |
| Thalamus | 5.2 (0.9 to 9.3)d | 1.1 (−7.4 to 9.3) | −10.0 (−14.4 to −1.5)d | −1.0 (−4.8 to 3.1) | −5.8 (−10.0 to −1.5)d |
Associations were studied using linear mixed effects models including a random intercept and slope. The estimated effect (95% CI) of the individual characteristics on CBF is presented. Of the a priori selected factors, the analysis of MAP and CBF was limited by insufficient power due to missing values and patient variation, and considered inconclusive. Hematocrit was associated with CBF only in one VOI.
No interaction with scan-order means that the effect of pCO2, temperature, and UF volume on CBF is similar at T1, T2, and T3. The pH model could be interpreted by adding the effect of the single term ‘pH’ and of the interaction term ‘pH*T2,’ yielding a net negative effect of pH on CBF at T2 as compared with T1.
P<0.01.
P<0.001.
P<0.05.
Associations of Cognitive Function and Structural Markers of Brain Lesions with CBF
No significant correlation between cognitive function or structural markers of brain lesions (i.e., the Fazekas score indicating severity of white matter lesions, and the presence of microbleeds) and baseline global or regional CBF was found (Supplemental Table 2).
Additionally, we tested the associations between cognitive function and structural markers of brain lesions with CBF using LMM, thereby including all CBF measurements. These analyses should be considered as hypothesis generating because of the relatively small sample size. Cognitive function and structural markers of brain lesions were not associated with global CBF (Supplemental Table 3). For regional CBF, a better executive function according to the Z-converted Trail Making Test B (TMT-B) and according to the TMT B/A ratio was associated with higher CBF at T2 as compared with T1 in several brain regions (Supplemental Table 3). A higher Fazekas score, indicating more severe white matter lesions, was associated with higher CBF in most regions at T2 as compared with T1. The presence of microbleeds was associated with higher CBF of the temporal lobe and cerebellum at T2 as compared with T1.
Adverse Event
One patient (identity 115) lost consciousness due to dialysis-induced hypotension shortly after the third scan. CBF decreased from 30.4 predialysis to 24.2 ml/100 g per minute (−20%) at T3 shortly before he lost consciousness. This patient made a full recovery without sequelae. None of the other patients experienced intradialytic hypotension (IDH, i.e., SBP<100 mm Hg, or IDH symptoms), or received any intervention for IDH during the HD study session.
Sensitivity Analyses
Because CBF changes in the left and right hemispheres did not differ significantly, the hemispheres were merged for the aforementioned VOIs analyses. The results were basically identical when both hemispheres were analyzed separately (Supplemental Table 4).
In the analysis with T2 as the reference point for CBF change, global and regional CBF declined significantly between T2 and T3 as well (Table 3). Global and regional CBF did not differ significantly between T1 and T2.
The HD-induced change in regional perfusion of the gray matter was analyzed separately as opposed to the combined gray and white matter perfusion of these regions. In all VOIs, the decline in gray matter perfusion was similar to or even greater than the sum of gray and white matter (Supplemental Table 5).
Discussion
The main finding of our study is that CBF declined by 10%±15% during a conventional HD session in elderly patients on maintenance HD. The decline in CBF was similar for the various individual brain regions that were studied and therefore, most likely, affected both the anterior (i.e., the internal carotid arteries) and posterior (i.e., the vertebral and basilar arteries) circulation. The decline in CBF (−20%) was symptomatic in one patient. HD treatment–related factors that might explain the intradialytic CBF decline were a higher tympanic temperature, a greater UF volume and UF rate, and a higher pH.
This study is new insofar as that CBF was quantitatively measured early and late during HD using a gold-standard technique, i.e., with [15O]H2O PET-CT scans. Previous studies estimating CBF during HD reported contradictory results and were limited by the use of the transcranial Doppler technique, which measures CBF velocity, and represents CBF only if the diameter of the insonated vessel remains constant during HD.23–28
Under normal physiologic conditions, CBF depends on cerebral perfusion pressure and cerebrovascular resistance. Hypothetically, CBF is kept relatively constant by cerebral autoregulation, a complex interplay of metabolic, myogenic, and neurogenic mechanisms. Whether HD affects these mechanisms due to the inherent hemodynamic stress and metabolic changes, is currently unknown. However, this study suggests that several HD treatment–related mechanisms might be involved in the intradialytic decline of CBF. First, cerebral perfusion pressure, defined as the difference between MAP and intracranial pressure, will depend largely on the MAP during HD. In this study, MAP decreased significantly between T2 and T3 but, unfortunately, the analyses of the association between MAP and CBF were inconclusive. Interestingly, a larger UF volume and rate, which may indicate greater hemodynamic stress, were associated with lower CBF. Second, cerebrovascular resistance might be modulated by intradialytic changes in metabolic factors, blood viscosity, and body temperature. pCO2, which was positively associated with CBF, remained constant during HD and did not explain the HD-induced CBF decline. A higher pH was associated with lower CBF only shortly after the start of HD, as compared with before the start of HD, but not at the end of HD. Hematocrit reflects blood viscosity, and an increase in hematocrit was reported to reduce CBF.29 In this study, the rise in hematocrit was very small and is unlikely to explain the decline in CBF. Finally, a higher tympanic temperature was associated with lower CBF, which is in accordance with a previous trial on dialysate cooling by Eldehni et al.15 These authors reported that lower dialysate temperature, which is thought to improve vascular resistance,30 led to improved hemodynamic stability and prevented the development of white matter lesions in patients with incident HD compared with the use of a dialysate temperature of 37.0°C.15,31 Notably, in this study we used a relatively low dialysate temperature (36.5°C) and kept the room temperature stable at 20°C. Even then, CBF declined significantly.
An important question is whether repetitive HD-induced CBF declines are causally related to ischemic brain lesions and cognitive decline. To our knowledge, no data are available on clinical effects of a similar intervention-related CBF decline. Generally, the CBF threshold for ischemia is considered as <10 ml/100 g per minute, and <20 ml/100 g per minute for the penumbra that surrounds an ischemic event, indicating severely ischemic but still viable brain tissue.32 In this study, these absolute CBF thresholds were not reached, because the lowest individual CBF level was 24.4 ml/100 g per minute at T3. However, whether CBF reductions lead to ischemia also depends on the duration of the CBF reduction, blood oxygenation, the efficacy of oxygen extraction, and capillary (dys)function.33 The importance of oxygenation was underscored by a recent study that showed that a relative drop of 15% in cerebral oxygenation during HD, defined as cerebral ischemia, was associated with decreased executive cognitive function at 12 months.34 Additionally, cerebral oxygenation was reported to be lower in patients receiving HD compared with patients receiving peritoneal dialysis, and with controls.35–37 Moreover, oxygen extraction fraction was lower in patients receiving HD compared with controls.38 An underlying reason for the lower oxygen extraction fraction might be the concept of capillary dysfunction, which was recently proposed as a source of stroke-like symptoms and cognitive decline.33 In capillary dysfunction, changes in capillary flow patterns can limit the oxygen extraction in tissue, thereby making tissue hypoxia possible even in the presence of adequate cerebral blood supply.33 Thus, a CBF decline together with low cerebral oxygenation, and low oxygenation extraction, might put the brain at risk for ischemia at a relatively higher CBF in patients receiving HD compared with nondialysis patients. Interestingly, endothelial injury and dysfunction, which is a common feature in patients receiving HD,39,40 is considered an important source of capillary dysfunction.33 In this study HD-induced endothelial dysfunction likely occurred because plasma levels of myeloperoxidase and pentraxin 3 rose significantly during HD.41–43
A limitation of our study is that we included a relatively small number of subjects due to the practical challenges of performing intradialytic PET-CT scans, especially in the elderly. Nevertheless, [15O]H2O PET is the gold standard to measure CBF and this is the first study that quantitatively studied CBF during HD. Another limitation is that we included only elderly patients, with a relatively long median dialysis vintage, thereby limiting generalizability of our findings to the general dialysis population. In light of the small sample size, our findings with respect to the secondary objective of this study, i.e., associations of HD treatment–related factors with CBF, should be considered with caution. Future studies with a larger cohort of patients are needed to evaluate the intradialytic course of CBF in relation to simultaneous changes of MAP, pH, temperature, and hematocrit, and UF volume and rate, because the identification of HD treatment–related factors involved in CBF decline might help guide the design of new HD protocols that minimize cerebrovascular stress. Additionally, the longitudinal association between HD-induced CBF declines and cognitive function needs further attention.
In conclusion, conventional HD induces a significant reduction in global and regional CBF in elderly patients receiving HD. Repetitive intradialytic decreases in CBF may be one of the mechanisms by which HD induces cerebral ischemic injury.
Concise Methods
Patients and Study Design
This study was performed according to the principles of the Declaration of Helsinki and was approved by the Medical Ethical Committee of the University Medical Center Groningen, and registered at clinicaltrials.gov (NCT02272985). All patients gave written informed consent. The study was performed between March and November of 2015.
Patients receiving HD aged ≥65 years from our department with an arteriovenous fistula without significant recirculation were eligible for this study. Patients were studied during a regular dialysis session after the longest interdialytic interval (Monday or Tuesday). Patient characteristics were assessed at study entry and retrieved from the patients’ medical history. Height was measured before, and weight before and after the HD study session. BP, heart rate, and tympanic temperature were measured before every PET-CT scan and every 30 minutes during the HD study session. For more information on study design, including additional in- and exclusion criteria, we refer to the Full Concise Methods (Supplemental Material).
HD Study Session
All HD study sessions were performed in the afternoon in the PET-camera room. The ambient temperature of the room was kept constant at 20°C, excluding an effect of outside temperature on cardiovascular stability during study sessions. After the first PET scan (T1), patients started dialysis still being in a horizontal position in the PET-camera. After the second PET scan (T2), which was performed within 30 minutes after the start of HD, patients were transferred to a hospital bed adjacent to the PET-camera to continue dialysis in a 30–45-degree supine position. Before the third PET scan (T3), which was performed in the final hour of the HD session. Approximately 30 minutes before the start of the third PET scan (T3), which was performed in the final hour of the HD session, patients were transferred back to the PET.
A low-dose brain computed tomography was made before the first and third PET scans to correct for attenuation of the PET data. A bolus injection of [15O]H2O was administered intravenously at a constant rate through an indwelling peripheral venous catheter in the non–dialysis access arm. The injected dose of [15O]H2O was 500 MBq per scan, with a total dose of 1500 MBq per patient for the whole study. During each PET scan, arterial blood was sampled continuously from the dialysis line by a dedicated programmable blood-sampler to obtain the course of the radioactivity concentration in the blood during 5 minutes after the injection of [15O]H2O. To perform laboratory measurements, arterial blood was sampled from the arterial dialysis line just before each PET scan.
Dialysis Settings
All patients were on bicarbonate dialysis with a low-flux polysulfone hollow-fiber dialyzer (F8; Fresenius Medical Care, Bad Homburg, Germany). Blood flow and dialysate flow rates were 200–300 and 500 ml/min, respectively. Dialysate temperature was 36.5°C in all patients. We used constant UF rate and dialysate conductivity. For dialysate composition we refer to the Full Concise Methods (Supplemental Material).
PET Data Acquisition
For the [15O]H2O PET-CT scans a Siemens Biograph 64-mCT (Siemens Medical Systems, TN) that acquires 109 planes over a total axial length of 216 mm was used. For details on the [15O]H2O production we refer to the Full Concise Methods (Supplemental Material).
First, a low-dose computed tomography scan was performed for attenuation and scatter correction. The dynamic PET acquisition (310 seconds) was started, followed after 10 seconds by an intravenous bolus injection of [15O]H2O. In total, the duration of every PET-CT scan was 5 minutes, which was uniform across all time points and all patients. Head movement was minimized with a head-restraining band. For CBF quantification, the arterial input function was obtained from arterial blood radioactivity, which was continuously monitored with an automated sampling system (Veenstra Instruments, Joure, The Netherlands). One extra blood sample was collected at 393±32 seconds after tracer injection to determine the amount of radioactivity in the blood using a γ-counter (Wizard2, Perkin Elmer, Waltham).
Three of the 36 scans could not be analyzed due to a technical problem with the automated sampling system during the measurement (patient identity 106 [T1], patient identity 107 [T2], patient identity 102 [T3]).
MRI Data Acquisition
MRI was performed using a 1.5T whole body system (Aera, Siemens, Erlangen, Germany) on a nondialysis day. The study MRI was performed median 3 days (range, −72 to +3 days) after the HD study session. The scan protocol (total scan time 30 minutes) included T1-weighted, T2-weighted, three-dimensional fluid-attenuated inversion recovery, diffusion-weighted imaging, susceptibility-weighted imaging, and two-dimensional phase contrast sequences. No intravenous contrast was used. A neuroradiologist (PJvL) assessed white matter hyperintensities and cortical atrophy, using the Fazekas scale and the global cortical atrophy scale, respectively.44,45 Microbleeds were scored on the susceptibility-weighted imaging sequence.
Image Reconstruction and Preprocessing
Image processing and pharmacokinetic analysis were performed with PMOD 3.8 software (PMOD Technologies Ltd., Zurich, Switzerland). The average image (time-weighted) was used for rigid matching registration of the individual PET to the individual MRI. See Full Concise Methods (Supplemental Material) for background information on image reconstruction and processing.
Neuropsychologic Tests
A neuropsychologic assessment battery was performed to characterize the study population and included all major cognitive domains. For details on the neuropsychologic assessment battery we refer to the Full Concise Methods (Supplemental Material). The order of the tests was fixed and cognitive testing was performed on a nondialysis day. It took approximately 45–60 minutes per subject to complete the tests. The neuropsychologic assessment was performed median 95 days (range, −196 to −33 days) before the HD study session.
Statistical Analyses
Intradialytic changes in levels of the HD-related characteristics were studied using repeated measures ANOVA (with a Greenhouse–Geisser correction in case of nonsphericity), with a Bonferroni correction.
For the primary study objective, global and regional CBF changes were analyzed by LMM, which allowed for individual random intercepts and slopes of CBF over time. The random slopes were on the basis of the actual scan times per patient. Relative CBF change was calculated as the mean of the individual percentual change between T1 and T3 using descriptive statistics, and is reported as mean±SD (%).
For the secondary study objective, associations of HD treatment–related HD treatment-related factors, which might potentially explain CBF change, with CBF were studied. Those factors included MAP, pCO2, pH, tympanic temperature, hematocrit, and UF volume and were selected on the basis of literature.15,23,24,26,34,46–48 The factors were studied univariately using LMM, checking the significance of interactions with scan-order. Because UF volume was associated with CBF, the association between UF rate and CBF was evaluated as well.
In additional analyses, associations of cognitive test scores and structural brain characteristics with CBF were explored. To this end, we first tested correlations with baseline CBF using Pearson or Spearman correlation, if appropriate. Subsequently, we studied the associations including all CBF measurements in an LMM. For these analyses, the cognitive test scores were converted to Z scores.
Several sensitivity analyses were performed. First, regional CBF change was also calculated for the left and right hemisphere separately. Second, in order to eliminate a possible effect of HD on the arterial sampling from the arteriovenous fistula, CBF change between T2 and T3 was calculated. Third, CBF change in only the gray matter of each VOI was studied instead of the sum of gray and white matter of the corresponding region.
Two-sided P<0.05 was considered statistically significant. Statistical analyses were performed with SPSS, version 23 (SPSS Inc., IBM company), GraphPad Prism version 5.0 (GraphPad Software, San Diego), and R version 3.4.0 (R Core Team, 2017).
Disclosures
None.
Supplementary Material
Acknowledgments
We want to thank the positron emission tomography technicians Yvonne van der Knaap, Eelco Severs, Paul van Snick, Johan Wiegers, and Aafke Zeilstra of the Medical Imaging Center, Department of Nuclear Medicine and Molecular Imaging at University Medical Center Groningen, The Netherlands for their technical support during the study sessions. Furthermore, we want to thank medical students Brandt Dijksterhuis, Thom Eshuis, Rozemarijn Ettema, Marleen Huberts, and Renske Wiersema for their help with the study sessions, and Lara Wagenaar for the performance of the neuropsychologic assessments.
This study was financed by a grant from the Healthy Aging Pilot Fund of the University Medical Center Groningen, The Netherlands (grant no. 2014-1/193).
The study was presented at the American Society of Nephrology Kidney Week, New Orleans, LA, November 2, 2017.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related perspective, “Filtering the Evidence: Is There a Cognitive Cost of Hemodialysis?,” on pages 1087–1089.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017101088/-/DCSupplemental.
References
- 1.Saran R, Robinson B, Abbott KC, Agodoa LY, Albertus P, Ayanian J, et al.: US renal data system 2016 annual data report: Epidemiology of kidney disease in the united states. Am J Kidney Dis 69[Suppl 1]: A7–A8, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Couser WG, Remuzzi G, Mendis S, Tonelli M: The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 80: 1258–1270, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Murray AM, Tupper DE, Knopman DS, Gilbertson DT, Pederson SL, Li S, et al.: Cognitive impairment in hemodialysis patients is common. Neurology 67: 216–223, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Sarnak MJ, Tighiouart H, Scott TM, Lou KV, Sorensen EP, Giang LM, et al.: Frequency of and risk factors for poor cognitive performance in hemodialysis patients. Neurology 80: 471–480, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurella Tamura M, Yaffe K: Dementia and cognitive impairment in ESRD: Diagnostic and therapeutic strategies. Kidney Int 79: 14–22, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray AM: Cognitive impairment in the aging dialysis and chronic kidney disease populations: An occult burden. Adv Chronic Kidney Dis 15: 123–132, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iyasere O, Okai D, Brown E: Cognitive function and advanced kidney disease: Longitudinal trends and impact on decision-making. Clin Kidney J 10: 89–94, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davey A, Elias MF, Robbins MA, Seliger SL, Dore GA: Decline in renal functioning is associated with longitudinal decline in global cognitive functioning, abstract reasoning and verbal memory. Nephrol Dial Transplant 28: 1810–1819, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drew DA, Weiner DE, Tighiouart H, Duncan S, Gupta A, Scott T, et al.: Cognitive decline and its risk factors in prevalent hemodialysis patients. Am J Kidney Dis 69: 780–787, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurella Tamura M, Vittinghoff E, Hsu CY, Tam K, Seliger SL, Sozio S, et al.; CRIC Study Investigators : Loss of executive function after dialysis initiation in adults with chronic kidney disease. Kidney Int 91: 948–953, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray AM, Seliger S, Lakshminarayan K, Herzog CA, Solid CA: Incidence of stroke before and after dialysis initiation in older patients. J Am Soc Nephrol 24: 1166–1173, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang R, Liu K, Yang L, Zhou T, Qian S, Li B, et al.: Reduced white matter integrity and cognitive deficits in maintenance hemodialysis ESRD patients: A diffusion-tensor study. Eur Radiol 25: 661–668, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Hsieh TJ, Chang JM, Chuang HY, Ko CH, Hsieh ML, Liu GC, et al.: End-stage renal disease: In vivo diffusion-tensor imaging of silent white matter damage. Radiology 252: 518–525, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Chou MC, Hsieh TJ, Lin YL, Hsieh YT, Li WZ, Chang JM, et al.: Widespread white matter alterations in patients with end-stage renal disease: A voxelwise diffusion tensor imaging study. AJNR Am J Neuroradiol 34: 1945–1951, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eldehni MT, Odudu A, McIntyre CW: Randomized clinical trial of dialysate cooling and effects on brain white matter. J Am Soc Nephrol 26: 957–965, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dasselaar JJ, Slart RH, Knip M, Pruim J, Tio RA, McIntyre CW, et al.: Haemodialysis is associated with a pronounced fall in myocardial perfusion. Nephrol Dial Transplant 24: 604–610, 2009 [DOI] [PubMed] [Google Scholar]
- 17.McIntyre CW, Burton JO, Selby NM, Leccisotti L, Korsheed S, Baker CS, et al.: Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol 3: 19–26, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selby NM, Lambie SH, Camici PG, Baker CS, McIntyre CW: Occurrence of regional left ventricular dysfunction in patients undergoing standard and biofeedback dialysis. Am J Kidney Dis 47: 830–841, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Burton JO, Jefferies HJ, Selby NM, McIntyre CW: Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol 4: 1925–1931, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang K, Herzog H, Mauler J, Filss C, Okell TW, Kops ER, et al.: Comparison of cerebral blood flow acquired by simultaneous [15O]water positron emission tomography and arterial spin labeling magnetic resonance imaging. J Cereb Blood Flow Metab 34: 1373–1380, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herscovitch P, Markham J, Raichle ME: Brain blood flow measured with intravenous H2(15)O. I. Theory and error analysis. J Nucl Med 24: 782–789, 1983 [PubMed] [Google Scholar]
- 22.Raichle ME, Martin WR, Herscovitch P, Mintun MA, Markham J: Brain blood flow measured with intravenous H2(15)O. II. Implementation and validation. J Nucl Med 24: 790–798, 1983 [PubMed] [Google Scholar]
- 23.Hata R, Matsumoto M, Handa N, Terakawa H, Sugitani Y, Kamada T: Effects of hemodialysis on cerebral circulation evaluated by transcranial Doppler ultrasonography. Stroke 25: 408–412, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Stefanidis I, Bach R, Mertens PR, Liakopoulos V, Liapi G, Mann H, et al.: Influence of hemodialysis on the mean blood flow velocity in the middle cerebral artery. Clin Nephrol 64: 129–137, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Skinner H, Mackaness C, Bedforth N, Mahajan R: Cerebral haemodynamics in patients with chronic renal failure: Effects of haemodialysis. Br J Anaesth 94: 203–205, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Metry G, Spittle M, Rahmati S, Giller C, Giller A, Kaufman A, et al.: Online monitoring of cerebral hemodynamics during hemodialysis. Am J Kidney Dis 40: 996–1004, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Regolisti G, Maggiore U, Cademartiri C, Cabassi A, Caiazza A, Tedeschi S, et al.: Cerebral blood flow decreases during intermittent hemodialysis in patients with acute kidney injury, but not in patients with end-stage renal disease. Nephrol Dial Transplant 28: 79–85, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Postiglione A, Faccenda F, Gallotta G, Rubba P, Federico S: Changes in middle cerebral artery blood velocity in uremic patients after hemodialysis. Stroke 22: 1508–1511, 1991 [DOI] [PubMed] [Google Scholar]
- 29.Metry G, Wikström B, Valind S, Sandhagen B, Linde T, Beshara S, et al.: Effect of normalization of hematocrit on brain circulation and metabolism in hemodialysis patients. J Am Soc Nephrol 10: 854–863, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Beerenhout CH, Noris M, Kooman JP, Porrati F, Binda E, Morigi M, et al.: Nitric oxide synthetic capacity in relation to dialysate temperature. Blood Purif 22: 203–209, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Chesterton LJ, Selby NM, Burton JO, McIntyre CW: Cool dialysate reduces asymptomatic intradialytic hypotension and increases baroreflex variability. Hemodial Int 13: 189–196, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Baron JC: Perfusion thresholds in human cerebral ischemia: Historical perspective and therapeutic implications. Cerebrovasc Dis 11[Suppl 1]: 2–8, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Østergaard L, Engedal TS, Moreton F, Hansen MB, Wardlaw JM, Dalkara T, et al.: Cerebral small vessel disease: Capillary pathways to stroke and cognitive decline. J Cereb Blood Flow Metab 36: 302–325, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacEwen C, Sutherland S, Daly J, Pugh C, Tarassenko L: Relationship between hypotension and cerebral ischemia during hemodialysis. J Am Soc Nephrol 28: 2511–2520, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoshino T, Ookawara S, Goto S, Miyazawa H, Ito K, Ueda Y, et al.: Evaluation of cerebral oxygenation in patients undergoing long-term hemodialysis. Nephron Clin Pract 126: 57–61, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Prohovnik I, Post J, Uribarri J, Lee H, Sandu O, Langhoff E: Cerebrovascular effects of hemodialysis in chronic kidney disease. J Cereb Blood Flow Metab 27: 1861–1869, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Papadopoulos G, Dounousi E, Papathanasiou A, Papathanakos G, Tzimas P: Cerebral oximetry values in dialyzed surgical patients: A comparison between hemodialysis and peritoneal dialysis. Ren Fail 35: 855–859, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Kanai H, Hirakata H, Nakane H, Fujii K, Hirakata E, Ibayashi S, et al.: Depressed cerebral oxygen metabolism in patients with chronic renal failure: A positron emission tomography study. Am J Kidney Dis 38[Suppl 1]: S129–S133, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Koç M, Bihorac A, Segal MS: Circulating endothelial cells as potential markers of the state of the endothelium in hemodialysis patients. Am J Kidney Dis 42: 704–712, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Koc M, Richards HB, Bihorac A, Ross EA, Schold JD, Segal MS: Circulating endothelial cells are associated with future vascular events in hemodialysis patients. Kidney Int 67: 1078–1083, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Stenvinkel P: Endothelial dysfunction and inflammation-is there a link? Nephrol Dial Transplant 16: 1968–1971, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Vita JA, Brennan ML, Gokce N, Mann SA, Goormastic M, Shishehbor MH, et al.: Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation 110: 1134–1139, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Witasp A, Rydén M, Carrero JJ, Qureshi AR, Nordfors L, Näslund E, et al.: Elevated circulating levels and tissue expression of pentraxin 3 in uremia: A reflection of endothelial dysfunction. PLoS One 8: e63493, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA: MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 149: 351–356, 1987 [DOI] [PubMed] [Google Scholar]
- 45.Scheltens P, Pasquier F, Weerts JG, Barkhof F, Leys D: Qualitative assessment of cerebral atrophy on MRI: Inter- and intra-observer reproducibility in dementia and normal aging. Eur Neurol 37: 95–99, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Vorstrup S, Lass P, Waldemar G, Brandi L, Schmidt JF, Johnsen A, et al.: Increased cerebral blood flow in anemic patients on long-term hemodialytic treatment. J Cereb Blood Flow Metab 12: 745–749, 1992 [DOI] [PubMed] [Google Scholar]
- 47.Farhoudi M, Abedi Azar S, Abdi R: Brain hemodynamics in patients with end-stage renal disease between hemodialysis sessions. Iran J Kidney Dis 6: 110–113, 2012 [PubMed] [Google Scholar]
- 48.Mathew RJ, Rabin P, Stone WJ, Wilson WH: Regional cerebral blood flow in dialysis encephalopathy and primary degenerative dementia. Kidney Int 28: 64–68, 1985 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.