Conventional in-center hemodialysis (HD) is the most common form of dialysis in the United States. Approximately 88% of individuals with ESRD initiate RRT with in-center HD.1 On the basis of current trends, in-center HD is anticipated to remain the dominant dialysis modality into the foreseeable future. The growing HD population is also ageing, with the highest incidence of ESRD in persons aged 75 years or older.1 As more patients of older age and/or high comorbidity initiate HD, awareness of the high burden of unfortunate side effects related to the HD treatment has increased, and emerging literature has demonstrated a high frequency of cognitive impairment and decline in cognition in the HD population.2,3 Cognitive impairment affects all aspects of wellbeing; it is associated with higher mortality, increased hospitalization rates, and lower quality of life. Furthermore, cognitive impairment can reduce medication adherence and dietary compliance that are central to the care of patients on dialysis. Severe cognitive impairment can impair decision-making capacity, creating a barrier to discussion of goals of care for this high-risk population. Although patients on dialysis have traditional and ESRD-specific risk factors for cognitive impairment, it is crucial to evaluate the role of the HD process itself in contributing to cognitive decline.
There is accumulating evidence that the HD process itself may accelerate cognitive decline. In a retrospective study, we compared the incidence of dementia in two ESRD populations: those initiated on HD and those initiated on peritoneal dialysis (PD).4 Despite adjustments for demographics and comorbidities and attempting to control for modality selection bias, we found a substantially higher incidence of dementia in those on HD compared with those on PD. In a prospective study, Iyasere et al.5 found that, over a median follow-up of 12 months, cognition declined faster in those on HD compared with PD, despite similar baseline cognitive scores and adjustment for education and demographics. The above studies have limitations including use of registry data that relies on diagnoses rather than actual cognitive testing in the large retrospective study, the small sample size in the prospective study (25 participants on PD and 41 participants on HD), and an inability to truly control for the cognitive differences that may lead to bias in dialysis modality selection. However, despite the limitations, the studies provide some evidence that it is not solely ESRD-specific factors that lead to cognitive decline, but perhaps the HD process itself. These findings suggest that an additional focus on HD-specific interventions may be an avenue to reduce the development or acceleration of cognitive impairment in patients on HD.
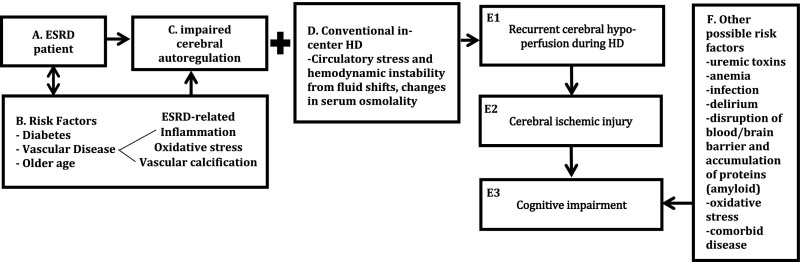
One area of focus is the potential for ischemic damage due to variations in blood flow that can occur during HD. In this issue of the Journal of the American Society of Nephrology, Polinder-Bos et al.6 report on the decline in intradialytic cerebral blood flow (CBF) that occurs in older adults on HD. The authors report a significant decline in CBF during HD that affected all regions of the brain and was surprisingly asymptomatic, except in one patient who experienced a 20% reduction in CBF and had a loss of consciousness. The authors conclude that these subclinical episodes of intradialytic cerebral hypoperfusion may contribute to the cerebral ischemic injury seen in patients on HD. Factors associated with intradialytic decline in CBF included higher ultrafiltration rate, higher body temperature, and higher blood pH. Although the association between intradialytic BP and CBF was inconclusive in this study, perhaps due to missing values and patient variation, the overall results support the growing literature that HD induces circulatory stress that can affect end-organs. It has been shown that intradialytic circulatory stress can result in cardiac stunning and accelerated loss of residual renal function. Like the kidneys, the brain has low resistance and high blood flow, rendering it particularly susceptible to ischemic damage. Although cerebral autoregulation resists changes in CBF, regulatory responses can be dysfunctional in patients on HD. Diabetes, hypertension, atherosclerotic disease, and older age can all impair cerebral autoregulation. In addition, ESRD-specific risk factors, including increased oxidative stress, inflammation, and vascular calcification from impaired calcium and phosphate regulation, can contribute to such impairment. The combination of hemodynamic instability during HD and diminished cerebral autoregulation can lead to cerebral hypoperfusion and subsequent ischemic injury (see Figure 1 for depiction of this process). Further supporting this hypothesis is an analysis of United States Renal Data System data that described a seven-fold increase in incident stroke rate during HD initiation that remained double the baseline risk 1 year later.7 Taken together, these studies suggest HD initiation and the HD process may lead to increased cerebral ischemic events and the resultant acceleration of cognitive impairment.
Figure 1.
Conceptual model describing a potential mechanism of cognitive decline due to HD-associated circulatory stress and cerebral hypoperfusion. Boxes A–C depict the risk factors common to patients on dialysis that can lead to impaired cerebral autoregulation. Pairing impaired cerebral autoregulation with the circulatory stress of conventional in-center HD (box D) can lead to downstream events shown in boxes E1–3, with cognitive impairment as the outcome. Box F describes the numerous other non-HD specific factors that can also contribute to cognitive impairment.
Building on the forgoing concepts, the next steps are to evaluate hemodynamic-centered interventions and utilize new methods to better characterize the pathophysiology of HD-related ischemic disease. Studies on intradialytic cerebral perfusion are limited by the methods used to monitor cerebral perfusion. Polinder-Bas et al. used the gold standard technique of [15O]H2O positron emission tomography-computed tomography scan to measure intradialytic CBF. However, the technical difficulties and expense of conducting HD during the scan contributed to their low sample size of 12 participants. Similarly, transcranial Doppler evaluation of cerebral perfusion is notorious for being operator dependent and personnel intensive. A newer technique, near-infrared spectroscopy (NIRS), is commonly used in clinical practice during carotid endarterectomy and coronary bypass surgery to monitor cerebral oximetry as a marker of cerebral ischemia. Cerebral oximetry, as measured by NIRS, is significantly correlated with change in middle cerebral artery blood flow.8 NIRS also provides the benefit of continuous monitoring throughout the dialysis session, ensuring that there are no missed episodes of cerebral ischemia between measurements. Advantages of NIRS also include ease of interpretation and set-up, lending NIRS well to implementation in clinical practice. A recent study used NIRS to examine the relationship between intradialytic BP, cerebral ischemia (quantified by changes in cerebral oximetry), and cognitive function.9 This study demonstrated intradialytic cerebral ischemia occurred frequently and correlated with decreased executive cognitive function. This provides more evidence that cerebral hypoperfusion can occur during HD, potentially leading to long-term cognitive decline. A larger study that harnesses the ease of use of the NIRS technology and includes both cerebral imaging and cognitive function would help confirm the association and clarify the mechanism.
With the significant evidence that HD may lead to cognitive decline, we face the daunting task of trying to fix conventional HD and improve intradialytic hemodynamic stability. A recent study indicated that use of cooled dialysate (set 0.5°C–1°C below body temperature) can reduce myocardial stunning.10 The same investigators also demonstrated that cooled dialysate improved systemic hemodynamic stability and reduced change in cerebral white matter.11 However, in the latter study of only 38 participants, the change in white matter in the standard temperature group was consistent with acute changes of ischemic insult, but not chronic injury. This may have been because of the timing of magnetic resonance imaging in relation to dialysis, but it certainly clouds interpretation of the results. Additional prospective studies are needed to confirm that cooled dialysate can prevent cerebral hypoperfusion and actually affect ischemic injury and prevent cognitive decline.
Although this is an important undertaking, we must remember the other options currently available for treating ESRD. PD, short frequent home dialysis, and nocturnal dialysis do not cause the significant hemodynamic stress that commonly accompanies conventional HD. The underutilization of these alternative dialytic techniques in the United States may have allowed the complications of HD to become more apparent, especially as older and sicker patients begin RRT with in-center HD. Use of these other dialysis alternatives, especially in patients at higher risk for cerebral hypoperfusion, may decrease the burden of cognitive impairment. However, this does challenge the nephrology community to be innovative and flexible in allowing more patients to utilize these alternatives and to create the infrastructure to support their successful implementation in older or chronically ill patients.
In short, to reduce cerebral ischemic injury and subsequent cognitive impairment, we must explore different paths by evaluating methods to reduce the circulatory stress of HD and simultaneously looking to augment the use of other dialysis modalities.
Disclosures
None.
Acknowledgments
The author thanks the Kendall family for their generous financial support of her research focused on older adults with kidney disease.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related article, “Hemodialysis Induces an Acute Decline in Cerebral Blood Flow in Elderly Patients,” on pages 1317–1325.
References
- 1.United States Renal Data System : 2017 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2017 [Google Scholar]
- 2.Murray AM, Tupper DE, Knopman DS, Gilbertson DT, Pederson SL, Li S, et al.: Cognitive impairment in hemodialysis patients is common. Neurology 67: 216–223, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Bossola M, Antocicco M, Di Stasio E, Ciciarelli C, Luciani G, Tazza L, et al.: Mini Mental State Examination over time in chronic hemodialysis patients. J Psychosom Res 71: 50–54, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Wolfgram DF, Szabo A, Murray AM, Whittle J: Risk of dementia in peritoneal dialysis patients compared with hemodialysis patients. Perit Dial Int 35: 189–198, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iyasere O, Okai D, Brown E: Cognitive function and advanced kidney disease: Longitudinal trends and impact on decision-making. Clin Kidney J 10: 89–94, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polinder-Bos HA, Vállez García D, Kuipers J, Elting JWJ, Aries MJH, Krijnen WP: Hemodialysis Induces an Acute Decline in Cerebral Blood Flow in Elderly Patients. J Am Soc Nephrol 29: 1317–1325, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray AM, Seliger S, Lakshminarayan K, Herzog CA, Solid CA: Incidence of stroke before and after dialysis initiation in older patients. J Am Soc Nephrol 24: 1166–1173, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Rawi PG, Smielewski P, Kirkpatrick PJ: Evaluation of a near-infrared spectrometer (NIRO 300) for the detection of intracranial oxygenation changes in the adult head. Stroke 32: 2492–2500, 2001 [DOI] [PubMed] [Google Scholar]
- 9.MacEwen C, Sutherland S, Daly J, Pugh C, Tarassenko L: Relationship between hypotension and cerebral ischemia during hemodialysis. J Am Soc Nephrol 28: 2511–2520, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jefferies HJ, Burton JO, McIntyre CW: Individualised dialysate temperature improves intradialytic haemodynamics and abrogates haemodialysis-induced myocardial stunning, without compromising tolerability. Blood Purif 32: 63–68, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Eldehni MT, Odudu A, McIntyre CW: Randomized clinical trial of dialysate cooling and effects on brain white matter. J Am Soc Nephrol 26: 957–965, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]