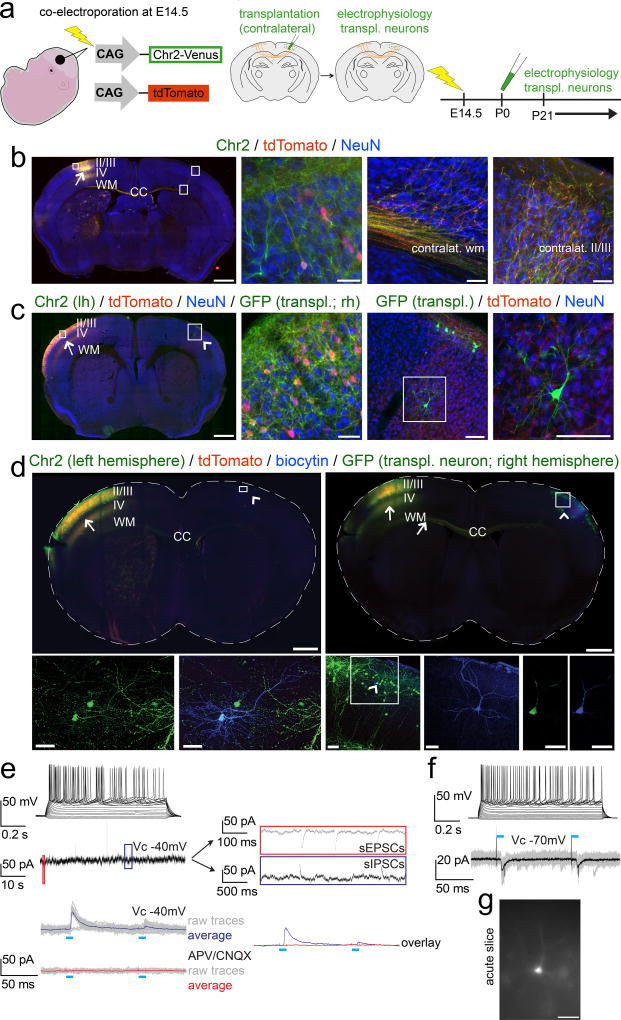
Figure 7. Newly incorporated neurons afferently integrate into long-distance trans-callosal circuitry.
(a) Schematic illustrating the experimental outline: in utero-electroporation of channelrhodopsin-2-Venus and tdTomato constructs into the left developing cortex at E14.5, targeting layer II/III CPN (left panel). Transplantation of eGFP+ neurons into the homotopic area of the contralateral cortex (identified by tdTomato fluorescence of CPN axon terminals) at P0/P1 (middle panel). Electrophysiology from 3 weeks of age on (right panel). (b) Epifluorescence montage of a coronal section after in utero-electroporation of layer II/III CPN with channelrhodopsin-2-Venus and tdTomato in the left hemisphere (arrow). The ipsilateral cortical area examined (box in left hemisphere) shows an electroporated neuron demonstrating membrane localization of channelrhodopsin-2-Venus in comparison to the cytoplasmic localization of tdTomato (high magnification panel 2). The boxed areas in the contralateral cortex, display the contralateral white and cortical gray matter, demonstrating that channelrhodopsin-2-Venus is also transported and localized to distal axonal compartments of in utero-electroporated CPN (high magnification panels 3 and 4). Immunocytochemistry for NeuN was performed to counterstain for neurons. (c) Analogous images are shown after additional transplantation of eGFP+ neurons into the contralateral hemisphere; the Venus signal was not amplified by ICC. The arrow indicates the electroporation site; the arrowhead points out the contralateral transplantation site. The boxed area in the left hemisphere is shown in high magnification in panel 2. A GFP+ transplanted neuron (note the strong native and cytoplasmic eGFP fluorescence) is situated in the boxed area in the right hemisphere and is shown in high magnification in panels 3 and 4. While the native Venus fluorescence is well visible in the membrane of neuronal somata and proximal neuronal processes within the electroporated left hemisphere, distal axonal compartments in vicinity of the transplanted neuron within the contralateral hemisphere exhibit only weak native Venus-fluorescence. However, the tdTomato signal is well visible even in distant trans-callosal axonal compartments of electroporated neurons, tdTomato+ axon terminals (originating from contralateral recipient-derived layer II/IIICPN) are in close proximity to the transplanted neuron, suggesting that transplanted neurons might get integrated into trans-callosal long-distance circuitry within the recipient cortex. Channelrhodopsin-2-Venus (green, membrane bound), tdTomato (red, cytoplasmic), eGFP (green, cytoplasmic), NeuN (blue). Scale bars: b, c (motages), 1000 µm; b (panels 2 – 4, 50 µm; c (panel 2), 50 µm; c (panels 3,4), 100 µm.
(d) Whole-cell recordings were performed from transplanted neurons in slices of in utero-electroporated recipient brains to investigate for integration into long-distance callosal circuitry. Data for 2 example neurons are shown. ICC analysis of the slices demonstrates that the in utero-electroporations with channelrhodopsin-2-Venus and tdTomato were strictly unilateral. Arrows indicate the electroporation sites and axons of electroporated recipient-derived layer II/III CPN. Numerous channelrhodopsin-2-Venus and tdTomato positive axons are crossing the corpus callosum (CC). Arrowheads indicate the transplantation sites contralateral to the in utero-electroporation sites. Recorded neurons were filled with a biocytin-containing intracellular solution and revealed with post hoc ICC. The boxed areas of the transplantation sites are magnified and shown as pseudo-confocal stacks (collapsed into one optical plane; high magnification panels 1 – 3), displaying double-positivity of recorded transplanted neurons for eGFP and biocytin; the arrowhead within the boxed area in high magnification panel 3 indicates a recorded transplanted neuron; the blue channel (biocytin) of the boxed area is further magnified in the adjacent panel 4, displaying the fully reconstructed morphology of the recorded neuron. The complete overlap of cytoplasmic eGFP and cytoplasmic biocytin in this neuron, is shown in single optical confocal sections (panels 5, 6). Channelrhodopsin-2-Venus (green; membrane bound), tdTomato (red, cytoplasmic), eGFP (green, cytoplasmic), biocytin (blue, cytoplasmic). Scale bars: d (montages), 1000 µm; d (pseudo-confocal stacks, panels 1, 2), 40 µm; d (pseudo-confocal stack, panel 3), 70 µm; d (high magnification panel 4 and confocal panels 5, 6), 40 µm.
(e, f, top panels) Depolarization of transplanted neurons by current injections through the patch pipette resulted in robust trains of action potentials confirming neuronal identity., as expected. Evaluation of intrinsic membrane properties demonstrated the development of a mature electrophysiological phenotype with hyperpolarized resting membrane potentials (Vrest: −73 mV and −76 mV), membrane resistance- (Rm: 78 MΩ and 117 MΩ) and whole cell capacity (Cm: 166 pF and 104 pF) parameters reflecting typical values of mature pyramidal neurons. (e, middle panel) The transplanted neuron displayed in (d, left panels) was clamped to a membrane potential VC = −40mV and was continuously monitored for spontaneous synaptic events. The red and the blue boxes are resolved next to the trace on a slower time scale, revealing spontaneous EPSCs and IPSCs, indicating local and/or long-distance synaptic integration of transplanted neurons into recipient circuitry.
(e, f, bottom panels) Photo-stimulation (blue bars) of axons of in utero-electroporated recipient-derived layer II/III CPN (localized in the contralateral hemisphere) was performed while transplanted neurons were recorded in Vc = −70 mV to monitor EPSCs, or in Vc = −40 mV to monitor EPSCs and IPSCs simultaneously. (e, bottom panel) The transplanted neuron shown in (d, left panels) exhibited light-evoked IPSCs only (raw traces of 20 trials in gray; averaged traces in blue). Abolishment of the light-evoked IPSCs upon application of the AMPA/kainate and NMDA receptor blockers CNQX and APV respectively (raw traces of 20 trials in gray; averaged traces in red), strongly suggested that they were driven by glutamatergic inputs from contralateral recipient-derived layer II/III CPN to local inhibitory interneurons. The overlay of the averaged traces before (blue) and after (red) application of the blockers is shown on the right next to the traces. (f, bottom panel) The transplanted neuron shown in (d, right panels) exhibited light-evoked EPSCs (raw traces of 20 trials in gray; averaged traces in black), suggesting that the transplanted neuron received potentially monosynaptic input from contralateral layer II/III recipient-derived CPN. Also note the paired-pulse depression of light-evoked PSCs, indicating short-term plasticity of this input. (g) Epifluorescence image of the same neuron during recording. Scale bar: g, 40 µm.