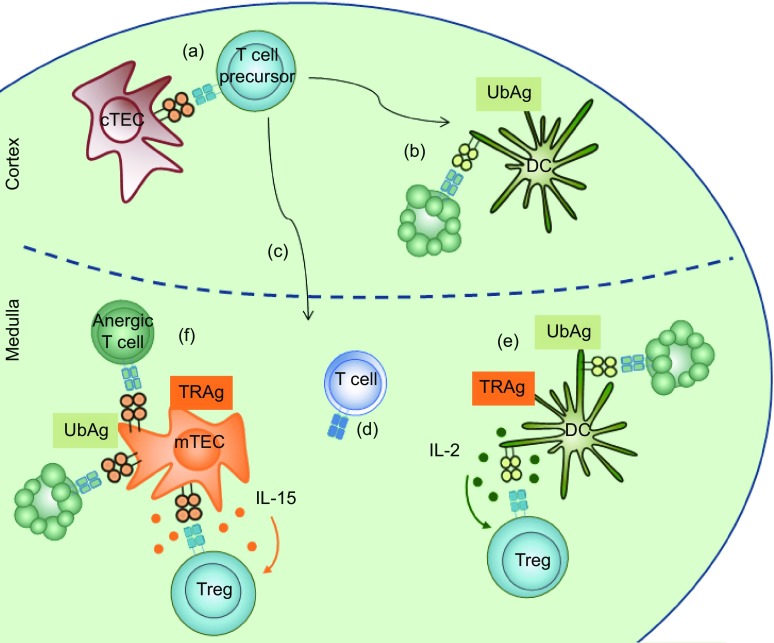
Figure 1.
A model for thymic development and selection of Treg. Rescue from programmed cell-death of probably common Tconv/Treg precursors requires interaction of their TCR with MHC/peptide complexes expressed on the surface of cortical thymic epithelial cells (cTEC) (a). The thus positively selected precursor-population undergoes a first wave of negative selection (i.e., induction of apoptosis) in the cortex, depleting it of cells specific for the “ubiquitous” antigens (UbAg) presented by cortical dendritic cells (DC) (b). Surviving thymocytes upregulate CCR7 and CCR4 and migrate to the medulla (c) where precursors that recognize MHC/self-peptide complexes with low affinity develop into fully mature Tconv (d). T cell-precursors that recognize MHC/self-peptide complexes with higher affinity have three distinct destinies; become anergic, die by apoptosis, or differentiate into Treg. The latter processes appear to especially concern cells specific for the peripheral-tissue restricted antigens (TRA) expressed by mTEC and presented by mTEC or, upon transfer, by thymic DC. The signals, that determine which of the three distinct outcomes a given autospecific T cell-precursor will adopt, remain incompletely identified, but the IL-2 produced by DC (e) and mTEC-derived IL-15 (f) drive precursors into the Treg lineage