Abstract
Coastal wetlands are important carbon sinks globally, but their ability to store carbon hinges on their nitrogen (N) supply and N uptake dynamics of dominant plant species. In terrestrial ecosystems, uptake of nitrate (NO3−) and ammonium (NH4+) through roots can strongly influence N acquisition rates and their responses to environmental factors such as rising atmospheric CO2 and eutrophication. We examined the 15N uptake kinetics of three dominant plant species in North American coastal wetlands (Spartina patens, C4 grass; Phragmites australis, C3 grass; Schoenoplectus americanus, C3 sedge) under ambient and elevated CO2 conditions. We further related our results to the productivity response of these species in two long-term field experiments. S. patens had the greatest uptake rates for NO3− and NH4+ under ambient conditions, suggesting that N uptake kinetics may underlie its strong productivity response to N in the field. Elevated CO2 increased NH4+ and NO3− uptake rates for S. patens, but had negative effects on NO3− uptake rates in P. australis and no effects on S. americanus. We suggest that N uptake kinetics may explain differences in plant community composition in coastal wetlands and that CO2-induced shifts, in combination with N proliferation, could alter ecosystem-scale productivity patterns of saltmarshes globally.
Introduction
Anthropogenic activities enrich the atmosphere with CO2, but the extent to which the biosphere can absorb this increase remains uncertain1,2. Saltmarshes play a disproportionally large role in global carbon storage, sequestering up to 87 Tg of carbon per year worldwide despite comprising less than 0.5% of the Earth’s land area3. However, the capacity of a given coastal wetland to store carbon hinges primarily on its nitrogen (N) supply and the N uptake dynamics of its dominant plant species4–6. In terrestrial ecosystems including coastal wetlands, root absorption of nitrate (NO3−) and ammonium (NH4+) strongly influences the rate of N acquisition by plants and how this varies in response to environmental factors7–9. Nitrogen availability also constrains ecosystem productivity, with N enrichment leading to species shifts that can favor both native10 and introduced species11,12. However, saltmarsh species differ in their N metabolizing activities, with rates influenced by the chemical form of N and its concentration in the substrate13–15, oxygenation of the rhizosphere16, salinity conditions17, and the presence of toxins such as sulfide18. Understanding the kinetics of root nitrogen uptake and potential differences among foundation species is therefore crucial for predicting saltmarsh ecosystems’ potential for carbon storage as atmospheric CO2 levels rise.
While the effects of elevated CO2 on plant productivity are well studied in a variety of ecosystems19,20 including saltmarshes21,22, our understanding of these responses is primarily based on changes in aboveground biomass, CO2 assimilation23,24, and shifts in species composition that reflect competition between plant functional groups10. Little attention has been paid to belowground mechanisms such as those associated with N acquisition or how they could be altered by global change. In an elevated CO2 environment, plants generally experience a decline in tissue N concentration25, due to either a dilution of Rubisco26–28 or a reduction of transpiration-driven mass flow of N through soils29; declines have been measured despite ample N supply30,31. Recent evidence also suggests that, at least for C3 plants under NO3− nutrition, CO2 enrichment can decrease tissue N directly by slowing growth and inhibiting shoot NO3− assimilation32. However, the direct effects of elevated CO2 on N uptake have only been investigated in a small number of studies, making it difficult to generalize how different species or functional groups may adjust N uptake in response to elevated CO2.
Although photosynthetic pathways typically determine plant physiological responses to elevated CO2, the circumstances under which these physiological differences translate into a change in N uptake kinetics is not yet clear. While N acquisition is generally not affected by elevated CO2 in C4 plants, C3 plants show variable patterns in uptake parameters under elevated CO2 (e.g., Vmax or Km)9. For example, in studies of temperate forest trees, the effect of elevated CO2 on NH4+ root uptake capacity was species dependent, ranging from +215% in Acer negundo to −40% in Quercus macrocarpa33. In related studies, elevated CO2 increased the maximum rate of NO3− uptake, specifically in Pinus ponderosa, Bouteloua eriopoda and Pinus taeda34–37. Other studies have found no significant effect of elevated CO2 on NO3− or NH4+ uptake rates, namely in Pinus taeda, Prosopis glandulosa, Ceratonia siliqua, and several herbaceous species35–40. Furthermore, effects of elevated CO2 are not limited to N uptake rates. In the case of a C3 tropical seagrass, Halodule uninervis, elevated CO2 inhibited NO3− assimilation and NO3− nutrition alone did not enhance the CO2 response41. One proposed explanation for the species-specific effects of elevated CO2 on NO3− assimilation in C3 plants is that elevated CO2 concentrations decrease photorespiration, thereby decreasing the amount of reductant (NADH) available to support NO3− reduction to NO2− in the first step of NO3− assimilation42. In contrast, the C4 carbon fixation pathway generates sufficient quantities of reductants in the cytoplasm of mesophyll cells, thus avoiding the inhibitive effect of elevated CO2 on NO3− assimilation43.
The physiological capacity for nitrogen uptake and assimilation may provide a key mechanistic explanation for interspecific differences in sensitivity to CO2 and N addition9. For example, N uptake dynamics may explain why N addition can favor coastal wetland species that do not respond strongly to elevated CO2 (i.e., Spartina patens), ultimately negating the enhanced productivity response at the ecosystem-level10. In the context of anthropogenically-induced changes to the carbon and nitrogen cycles in wetland ecosystems, information on physiological responses of N uptake to elevated CO2 could be highly relevant for understanding these species shifts and how they influence critical ecosystem-level phenomena such as resilience to sea level rise and carbon sequestration44.
Functional taxonomic groups are often linked with suites of traits, allowing for an extrapolation of results beyond a particular ecosystem. Herbaceous-dominated systems such as grasslands, deserts, tundra, and marshes are often dominated by distinct functional groups (e.g., C3 grasses), and traits associated with these functional groups may influence ecosystem-scale responses, such as shifts in net primary productivity and carbon sequestration, to interacting global change factors10. Coastal saltmarshes in North America are typically dominated by C4 grasses (e.g., Spartina patens (Aiton) Muhl.) or C3 sedges (e.g., Schoenoplectus americanus (Pers.) Volkart ex Schinz & R. Keller). However, an introduced lineage of the C3 grass Phragmites australis (Cav.) Trin. ex Steud. (common reed) is invading coastal and other wetlands throughout North America45, likely altering their response to global change22.
We investigated the N uptake kinetics of three functionally distinct foundation plant species in North American coastal wetlands under ambient and elevated CO2 conditions, and related these results to the growth of each species in response to global change factors with data from long-term in situ experiments. Specifically, we asked three questions:
Does N uptake capacity differ between Phragmites australis, Schoenoplectus americanus, and Spartina patens? Plants adapted to low nutrient environments typically invest considerably in belowground organs and consequently do not have a high maximum uptake capacity46. Given that S. patens invests in belowground organs to a lesser extent than P. australis and S. americanus, we predicted that it would have a higher maximum uptake capacity.
Does elevated CO2 affect N uptake kinetics? Given the decline in tissue N status observed in many plants grown under elevated CO2 despite adequate N supply, we predicted that elevated CO2 would negatively affect the uptake kinetics of NO3− and NH4+ in all three species.
Do N uptake kinetics of our species explain observations in the field? We hypothesized that patterns in N uptake kinetics would correspond to species’ productivity responses to both CO2 and N in long-term field experiments.
Results
Kinetics of NO3− and NH4+ uptake
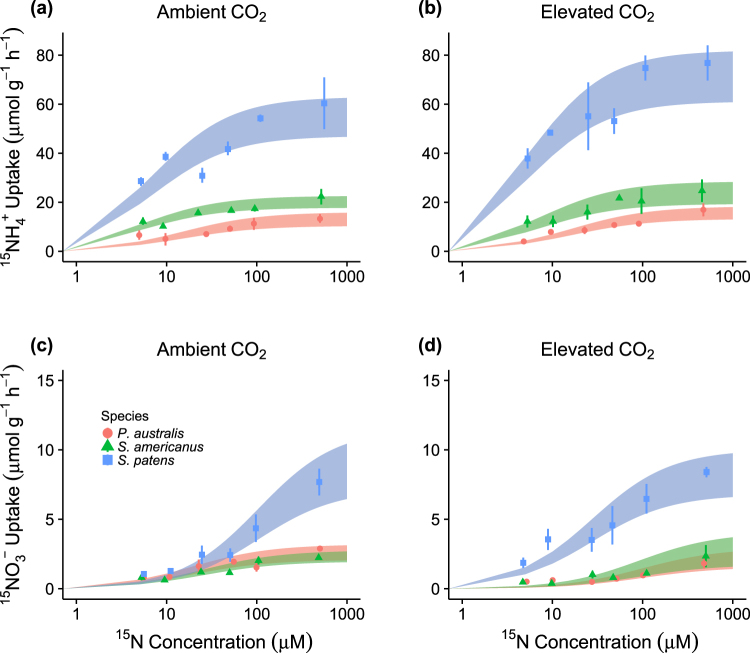
We performed a series of 15N uptake assays to test the hypothesis that saltmarsh species from contrasting functional groups (C4 grasses, C3 sedges, and C3 grasses) would differ in their N uptake characteristics. In a semi-controlled outdoor setting, we presented clonally propagated plants with varying concentrations of either 15NO3− or 15NH4+ and measured rates of N uptake by their root systems. All three species showed curvilinear relationships between N uptake rate (Vuptake) and N concentration that closely adhered to Michaelis-Menten reaction kinetics (Fig. 1). Moreover, all three species exhibited substantially greater Vuptake for NH4+ than NO3−, with rates differing by up to a factor of 10.
Figure 1.
Rates of 15N uptake by Spartina patens, Phragmites australis, and Schoenoplectus americanus during assays. Points are means (±SE) for replicate plants at the six N concentrations used; horizontal jitter has been added to reduce overlap. Shaded bands show the range of Michaelis-Menten curves corresponding to the bootstrapped 95% confidence interval for Vmax.
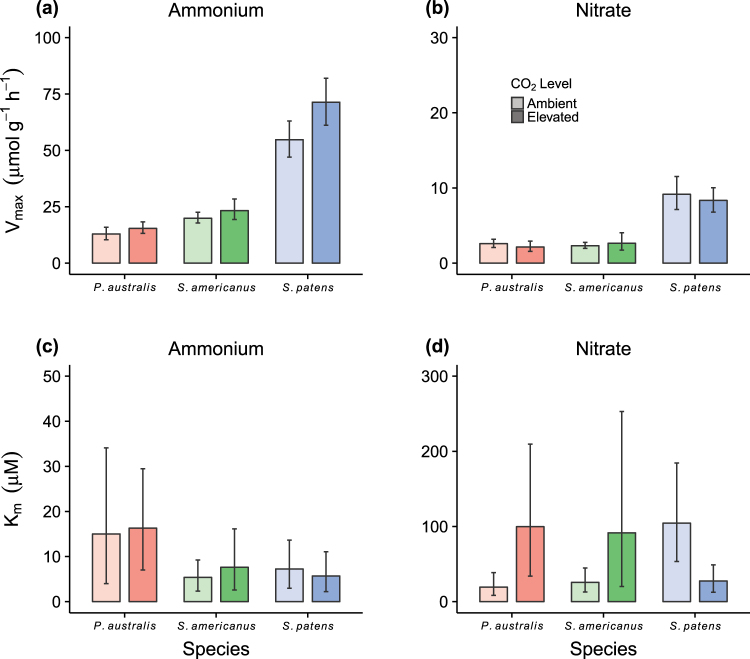
There were interspecific differences in Vuptake for both NH4+ and NO3− (Table 1). S. patens was primarily responsible for these differences, as it exhibited mean uptake rates up to 3 times greater than those of P. australis or S. americanus (Fig. 1a,c) and separated from both species in pairwise comparisons (Table 2). In addition, for NH4+, S. americanus had 20–30% greater mean Vuptake across the range of N concentrations than did P. australis (Fig. 1a). These interspecific differences also manifested in the parameter Vmax, the maximal uptake rate, when Michaelis-Menten curves were fit to the data; in this context, Vmax reflects a species’ capacity for N uptake under saturating N conditions. Using bootstrapped 95% confidence intervals (CIs), we again found that S. patens had greater Vmax than either of the C3 species for both NO3− and NH4+ (Fig. 2a,b), and that S. americanus had a greater Vmax for NH4+ than did P. australis (Fig. 2a).
Table 1.
Results of linear modeling analysis for nitrogen uptake (Vuptake).
| Model | Term | d.f. | F | P |
|---|---|---|---|---|
| NH 4 + uptake | Species | 2 | 278.38 | <0.001 |
| CO 2 | 1 | 10.44 | 0.002 | |
| N Conc | 1 | 26.17 | <0.001 | |
| Species × CO2 | 2 | 3.97 | 0.02 | |
| Species × N Conc | 2 | 1.74 | 0.18 | |
| CO2 × N Conc | 1 | 0.18 | 0.68 | |
| Species × CO2 × N Conc | 2 | 0.06 | 0.93 | |
| NO 3 − uptake | Species | 2 | 87.78 | <0.001 |
| CO2 | 1 | 0.05 | 0.83 | |
| N Conc | 1 | 111.94 | <0.001 | |
| Species × CO 2 | 2 | 15.26 | <0.001 | |
| Species × N Conc | 2 | 8.42 | <0.001 | |
| CO2 × N Conc | 1 | 0.31 | 0.58 | |
| Species × CO2 × N Conc | 2 | 0.82 | 0.44 |
Table 2.
Mean rates of inorganic N uptake (Vuptake; µmol g−1 h−1) across N concentrations by the three focal species. Group letters that differ within an N form denote statistical separation in pairwise comparisons of means.
| Species | CO 2 Level | NH 4 + | NO 3 − | ||||
|---|---|---|---|---|---|---|---|
| Mean | SE | Group | Mean | SE | Group | ||
| P. australis | Ambient | 9.09 | 2.11 | a | 1.60 | 0.24 | b |
| Elevated | 10.29 | 2.04 | a | 0.88 | 0.27 | a | |
| S. americanus | Ambient | 16.12 | 2.04 | b | 1.36 | 0.24 | ab |
| Elevated | 17.76 | 1.98 | b | 1.06 | 0.25 | ab | |
| S. patens | Ambient | 42.91 | 1.98 | c | 3.30 | 0.24 | c |
| Elevated | 57.34 | 2.04 | d | 4.81 | 0.24 | d | |
Figure 2.
Median of bootstrapped estimates for the Michaelis-Menten parameters Vmax and Km. Error bars depict the central 95% of estimates from across all bootstrapped fits (n = 999).
We carried out an additional set of assays under elevated CO2 to determine how the N uptake kinetics of our focal species could shift under future atmospheric conditions. We found that elevated CO2 altered patterns of N uptake, though these effects were species-specific and differed by N form and concentration (Table 1, Figs 1 and 2). For NH4+, mean Vuptake increased under elevated vs. ambient CO2, with S. patens showing the greatest, and statistically unequivocal, increases; the other two species exhibited non-significant trends towards increases as well (Fig. 1, Table 2). Also, S. patens continued to have greater Vuptake and Vmax for both NH4+ and NO3− than both C3 species when grown under elevated CO2, and the separation between S. americanus and P. australis in these metrics was maintained under elevated CO2 (Fig. 2a,b, Table 2). However, for NO3−, elevated CO2 induced a reduction in mean Vuptake for P. australis, such that it was not differentiable from S. americanus in either CO2 setting (Fig. 1c vs. d, Table 2).
For NO3−, interspecific differences in Vuptake depended on N concentrations and the CO2 level (Table 2), with CO2 inducing larger shifts within species at low N concentrations (Fig. 1c,d). Correspondingly, there was no evidence of CO2 affecting Vmax in any species, whereas it induced notable shifts in the Michaelis-Menten parameter Km (Fig. 2d). In the context of this study, Km reflects a species’ affinity for an N form, with smaller values indicative of greater affinity. The shift in Km under elevated CO2 was again greatest (and statistically unequivocal) for S. patens; bootstrapped CIs did not overlap. S. patens thus had a greater affinity for NO3− under elevated CO2 (Fig. 2d). Although the corresponding 95% CIs for P. australis were partly overlapping and the three-way interaction was not significant for Vuptake (Table 1), our data were consistent with P. australis experiencing the opposite shift, namely a decrease in affinity (i.e., an increase in Km) for NO3− under elevated CO2 (Fig. 2d). The data were likewise statistically equivocal for S. americanus (i.e., CIs were overlapping), as were CIs for all species with respect to NH4+ (Fig. 2c).
Growth responses to global change factors
To determine if N uptake kinetics can explain species responses to inorganic N eutrophication in the field, we compared the results of our assays with data on biomass production (aboveground and rhizome) from two long-term field experiments in which elevated CO2 and NH4+ were added factorially to plots in a Chesapeake Bay saltmarsh10,22. In the first five years of these experiments’ lifespans, N enrichment positively stimulated aboveground biomass production by S. patens and P. australis (by 214 and 220 g m−2, respectively; Fig. 3), with stimulation defined as the absolute difference in productivity between treatment and control conditions. In contrast, S. americanus responded negatively to N enrichment (−61 ± 27 g m−2; mean ± SE). Elevated CO2 positively stimulated aboveground biomass production for all three species, with the inter-annual mean change being greatest for P. australis (82 ± 26 g m−2), intermediate for S. americanus (20 ± 7 g m−2), and smallest for S. patens (7 ± 1 g m−2; Fig. 3a). Responses to the combined treatment (elevated CO2 + N) were likewise greatest for P. australis and S. americanus; they produced 202 and 161 g m−2 more than they did under the control, respectively (Fig. 3a). Belowground, P. australis also had the strongest growth responses to all three treatments, with the largest mean stimulation to rhizome biomass production observed under elevated CO2 + N (445 ± 175 g m−2, Fig. 3). N enrichment did not affect rhizome biomass stimulation of S. americanus (−10 ± 28 g m−2) and S. patens responded more strongly belowground to N enrichment (18 ± 7 g m−2) than to the other two treatments (Fig. 3).
Figure 3.
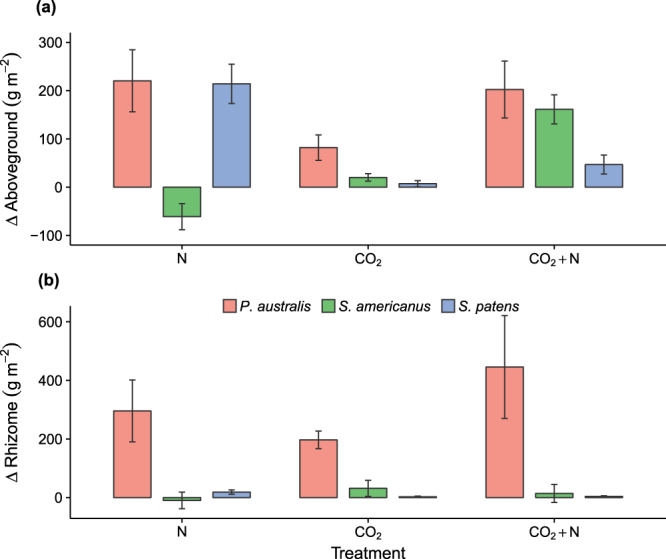
Mean (±SE) stimulation effects (i.e., difference from ambient experimental treatments) for (a) aboveground biomass production and (b) rhizome biomass production in experimental plots Global Change Research Wetland. The three treatments were CO2, elevated atmospheric CO2; N, nitrogen fertilization; and CO2 + N, both elevated CO2 and N fertilization. Means are calculated using the first 5 years’ worth of data from two long-term field experiments (see text).
Discussion
Our results suggest that plant responses to interacting global change factors may be related to differences in N acquisition kinetics among plant functional groups. In a prior analysis of the native saltmarsh community’s response to CO2 and N at our site, C4 grasses respond strongly to N addition10, demonstrating that N-induced plant community shifts can alter the ecosystem’s productivity response to elevated CO2. Our data suggest that this shift may be attributable to a difference in the N uptake capacity of the dominant C3 and C4 species in the community (S. americanus and S. patens, respectively). A high capacity for nutrient uptake, Vmax, is considered to be an adaptation to nutrient rich conditions, whilst a low Km denotes a high affinity for the substrate7. Here, Vmax levels for NH4+ uptake under ambient conditions were 150% higher in S. patens than in S. americanus, indicating that it is a high-nutrient species capable of taking advantage of N enrichment (Figs 1–3). In contrast, S. americanus is a low nutrient specialist (evidenced by low Vmax and low Km), and has a limited ability to take advantage of increased soil N (Fig. 3). Furthermore, plant species with a high Vmax generally do not produce a high root length density and are therefore competitively inferior when nutrients in soil solution are chronically low46. Consistent with this pattern, fine root production was, on average, twice as high in stands of S. americanus than in stands of S. patens over the past 20 years23. Given this, the divergent response of these two North American wetland species to elevated N is likely attributable to differences in their N uptake kinetics, and can therefore be used in a predictive framework to project plant community shifts in response to global change.
Plasticity in N uptake physiology may explain the ability of P. australis to thrive in both resource-poor and resource-rich habitats. For example, our results and those of a prior study47 suggest that P. australis is adapted to a low N environment, given its low Vmax. However, intermediate Vmax levels have been measured in P. australis13,48, as have levels an order of magnitude greater than we found49. As suggested by Romero et al.49, the ammonium uptake kinetics of P. australis seem to be plastic, such that they can be modified in response to nutrient availability or CO2 availability. The plastic response of N uptake to varying CO2 and N levels in the field study may partly explain why introduced P. australis can thrive under both high and low nutrient environments50. Our results provide evidence that P. australis has the kinetic parameters needed to invade low nitrogen environments, whilst our long-term field study shows that the species can thrive in resource rich environments. Furthermore, our long-term study clearly shows that P. australis can take advantage of both CO2 and N, with aboveground biomass stimulated most strongly by N addition, and belowground biomass stimulated most strongly by CO2 + N (Fig. 3).
Elevated CO2 affected saltmarsh functional groups differently. Both C3 species (P. australis and S. americanus) exhibited a trend for lower affinity for NO3− under elevated CO2 conditions, evidenced by increases in Km values. The functional group-specific effect of elevated CO2 on NO3− uptake capacity corresponds to differences in NO3− assimilation previously reported by Bloom et al.32,43 and may be attributable to the reduction in photorespiration that C3 plants experience under elevated CO2 conditions, as this decreases the reductant available to power the first step of NO3−assimilation42. Conversely, evidence of this repression was not observed in the C4 species, for which Km values actually decreased under elevated CO2. Again, elevated CO2 conditions appeared to reduce NO3− assimilation in C3, but not C4, plants.
It is now well established that the active process involved in ion uptake by plant roots at relatively low nutrient levels (10–200 µM) is provided by the high affinity transport system (HATS)8,51,52. This transport system is used by plants growing in natural and semi-natural ecosystems9,53 and is likely the one operating at the concentrations observed in our field experiment54. The HATS for both NO3− and NH4+ is subject to regulation in response to changes in external N availability or in the N demand of the whole plant55. However, the mechanisms underlying the suppression of N uptake under elevated CO2 remain unclear.
We found that elevated CO2 enhanced the physiological capabilities of our C4 species, such as increasing Vuptake of NH4+. This suggests that some C4 plants may become more competitive for N with near-future global change. Furthermore, this taxa-specific nutrient uptake response to elevated CO2 may influence differences in growth rate, as rates of N acquisition are often positively correlated with growth rates56–58. Indeed, S. patens had the kinetic parameters of an exploitative, fast growing species59, and data from the long-term field experiment show that its shoot biomass response to elevated N was on par with that of the invasive species P. australis. These physiological enhancements induced by elevated CO2 may also explain the stimulation effects of CO2 observed in C4 species at a 30 year experiment at our field site23,60. However, rapid NH4+ uptake does not necessarily translate into rapid growth; Zerihun & BassiriRad33 found that the relative growth rate of Acer negundo was unaffected by high CO2 despite experiencing a two-fold increase in root NH4+ uptake capacity in response to high CO2.
Elevated CO2 appeared to repress NH4+ uptake affinity in our dominant C3 species; this trend may help explain long-term observations in our field experiment. The increasing Km values for NH4+ under elevated CO2 could partly explain the reduction of tissue N levels experienced by foundational saltmarsh plants60. In some species, such as wheat, the reduction occurs despite the supply of high doses of nitrogen30 indicating that the observed reduction in tissue nitrogen with elevated CO2 is not due to a low nitrogen concentration in the root medium, but is related to aspects of uptake itself. Indeed, N addition did not sustain the initial positive CO2 stimulation of C3 biomass in one of our in situ experiments10. This was partially explained by competition with S. patens, but may also be attributable to sustained CO2 enrichment having a gradual decreasing effect on NH4+ uptake capacity. In addition, the combined effects of N and CO2 on P. australis shoot biomass was smaller than the effect of N alone, suggesting a potential negative effect. Another contributing factor may indeed be the reduction of transpiration-driven mass flow of N through soils due to a reduction in stomatal conductance usually experienced by plants under elevated CO2 conditions29. Alternatively, the pattern may derive from reductions in root respiration, given that greater tissue N content entails greater maintenance respiration61 and the fact that the energy requirements for NH4+ and NO3− uptake and assimilation constitute a significant portion of root respiration62. Reductions in root respiration as a result of elevated CO2 exposure have been reported in the literature63 but no satisfactory mechanisms to explain these effects have been demonstrated64,65.
As is the case for NO3− uptake, the mechanisms underlying the suppression of NH4+ uptake under elevated CO2 remain uncertain. What is clear is that carbon metabolites such as glutamine can suppress the expression of genes associated with the HATS for both NO3− and NH4+66–68. Additionally, glucose supply to plant roots can inhibit the induction of some enzymatic proteins such as glutamate dehydrogenase and asparagine synthetase69, both of which are involved in N metabolism70. The fact that C and N metabolism are tightly linked is inescapable71, and it may be the case that increased carbohydrate supply to roots as a result of elevated CO2 exposure may act directly or indirectly on plant nitrogen pools, ultimately causing a downregulation of genes associated with N uptake.
The extent to which N uptake is influenced by edaphic factors such as oxygenation of the rhizosphere, salinity conditions, or sulfide concentration was not investigated in this study. To evaluate uptake free of these effects, assays were conducted in aerobic solutions free from Na or hydrogen sulfide. Salinity is known to inhibit N uptake in Spartina alterniflora and P. australis by up to 40%13,17,48, especially at levels above 20 ppt. Similarly, anoxic conditions and hydrogen sulfide can inhibit N uptake18,48. How these factors influence N uptake in the field is unknown, although S. americanus has the ability to oxygenate its rhizosphere and can tolerate frequent flooding whilst S. patens inhabits higher saltier zones72. Therefore each species is specifically adapted to tolerate one of these confounding factors.
The interspecific differences in N uptake kinetics identified here provide an explanation for how individual plant-level responses to global change factors (such as CO2 and N enrichment) translate into species dynamics at a community level. We suggest that the ecosystem-level response to interacting global change factors can be related to the root uptake kinetics of N acquisition by different plant functional groups. Our results further demonstrate that P. australis is capable of invading low nitrogen ecosystems, whilst our long-term field study shows that it can also thrive in resource rich environments. Consequently, physiological plasticity in the invasive species appears to facilitate its proliferation. Further study is required to determine if rising atmospheric CO2 levels can be expected to repress N uptake in other ecosystems and to examine the specific mechanisms involved.
Methods
Nitrogen uptake assays
Three wetland taxa were selected for this study: Schoenoplectus americanus, Spartina patens, and a lineage of Phragmites australis subsp. australis (haplotype M). All three are highly abundant in saltmarshes along the Atlantic Coast of North America and are representative of the plant functional groups that dominate tidal marshes, namely C3 sedges, C4 grasses, and C3 grasses, respectively. P. australis subsp. australis is both introduced and invasive in North America73, while the two natives are dominant species in two long-term experiments situated at the Smithsonian Environmental Research Center (SERC) in Maryland, USA.
The nutrient uptake experiment was conducted in a set of six chambers (1.0 × 0.7 × 1.0 m) located at Bryn Mawr College in Pennsylvania, USA (40.0297°N, 75.3139°W). During the course of the experiment, plants experienced natural temperature fluctuations, with a mean daily high of 29.8 ± 0.8 °C and a mean daily low of 19.5 ± 0.5 °C. The chambers had closed walls constructed of Lexan polycarbonate, though they were not air-tight. Blowers continuously moved air into chambers at a rate that replaced the volume of each chamber once approximately every two minutes. Three chambers were maintained at ambient CO2 and three at elevated CO2 (ambient +300 ppmv CO2). CO2 concentrations in the chambers were monitored with CM-0212 CO2 loggers (CO2 Meter, Ormond Beach, USA) and adjusted manually on a daily basis.
Plant material was collected in the spring of 2012 from SERC, maintained for one year in the Bryn Mawr College greenhouse, and propagated from rhizome fragments or emergent shoots in May 2013. Propagules were washed clean of organic matter and dead root material, and individual shoots were placed in square pots (10 cm sides) filled with clean sand to facilitate transfer to a hydroponic medium during N uptake assays. Thirteen plants per species were placed in each chamber in June 2013 (n = 234 total plants) and fertilized weekly with a 1/10th strength Hoaglands solution. Within 10 weeks, individual plants achieved a root mass suitable for assays (>100 mg dw).
To investigate NH4+ and NO3− uptake kinetics, we presented individual plants with a 15N-labeled substrate in hydroponic solution. The protocol for assays was adapted from Epstein et al.74 and Mozdzer et al.13. Briefly, plants were washed free of sand and placed in an N-free solution of 0.50 mM CaCl2 overnight to maintain root epidermal cell integrity. After equilibration, each plant was exposed to one of six different N concentrations (5, 10, 25, 50, 100, and 500 μΜ) of either 15NH4Cl or K15NO3, respectively (99% enriched; Cambridge Isotope Laboratories, Andover, USA) for 45 minutes in a well-mixed 0.50 mM CaCl2 solution. To ensure that drawdown would not exceed 10% of the starting concentration, the reaction volume for assays was adjusted to 2500 ml for the lowest two concentrations and 1000 ml for the remaining concentrations. The treatment assay solution was identical to the equilibration medium but contained the labeled N dose. Each exposure series for both forms of N was applied to the three species in each chamber, such that the complete set of assays was performed in triplicate (n = 216 plants). One additional plant per species from each chamber was exposed only to the equilibration medium as a control (n = 18 plants). After 45 minutes of exposure, roots were rinsed for 2 min with 1 mM KCl to remove any excess labeled substrate from root surfaces. Each plant was then separated into root, rhizome, and stem tissue and dried at 60 °C to constant weight. Dry tissue was ground using a Retsch Mixer Mill 400 (Verder Scientific, Haan, Germany). To minimize potential effects of diurnal variation in nutrient uptake, assays were conducted at approximately the same time each day (1000–1200 h) over the course of three weeks, with the three exposure series for one plant species, one N form, and one CO2 level (n = 18 plants) completed per day. Samples of root tissue were analyzed for 15N using a Europa Integra continuous flow mass spectrometer (UC Davis Stable Isotope Facility).
Uptake rates of 15N (Vuptake) for individual plants were calculated from the mass of 15N that they assimilated (massim, in µg)13,75:
| 1 |
| 2 |
where m1 is the mass of N in the sample (in μg), APEsamp is the atom % excess 15N of the root sample exposed to a labeled substrate, APEctrl is the atom % excess 15N in the control root sample, APEtreat is the atom % excess of the labeled 15N treatment, MW is the molecular weight of the N isotope, m2 is the dry root mass of the sample (in grams), and texp is the duration of the exposure to labeled substrate (in minutes). Several uptake rates were anomalously high, especially at low N concentrations (5–25 μM). This was probably due to carryover during mass spectrometry, so Vuptake values that were greater than those at both of the next two higher N concentrations within a series were omitted (n = 19).
The 15N uptake rates from each exposure series (n = 14–18 plants) were then fit to the Michaelis-Menten equation in order to derive values of maximal uptake rate (Vmax) and the substrate concentration at which the rate is 50% of Vmax (Km):
| 3 |
where [c] is the concentration of NH4+ or NO3−. Vmax (in µmol 15N g−1 h−1) provides a measure of uptake capacity under saturating N conditions, while Km (in µM of NH4+ or NO3−) provides an estimate of the species’ affinity for NH4+ or NO3−; smaller values correspond to greater affinity. Curve fitting was carried out in R using a self-starting non-linear regression function (SSmicmen from the nlstools library). To determine if there were differences among species and/or CO2 levels, we used bootstrapping to compute 95% confidence intervals for all parameter estimates (via nlsBoot, also from nlstools; n = 999 iterations). Estimates were considered different if there was no overlap between pairs of bootstrapped 95% confidence intervals76.
Linear models were used to determine how experimental factors (CO2 level, plant species, and N concentration) affected N uptake rates (Vuptake), with separate models fit to data for NO3− and NH4+. Both models had the same form, with species and CO2 level included as categorical variables but N concentration included as a continuous variable. Terms for all possible two and three way interactions were also included. Vuptake values were square root transformed to ensure residual normality. Tukey-adjusted pairwise comparisons were subsequently made among all species-CO2 level combinations; the family-wise error rate was held at 0.05. All statistical analyses were conducted in R version 3.2.3.
Long-term field experiments
We compared results from our ex-situ kinetic experiment with in situ data from two long-term experiments situated in a brackish tidal marsh within SERC’s Global Change Research Wetland (Kirkpatrick Marsh; 38.8742° N, 76.5474°W) in Edgewater, Maryland, USA. The first experiment was established in 2006 and examines the effect of elevated CO2 and mineral N addition on the dominant native saltmarsh species S. americanus and S. patens. The second study was established in 2011 and examines the effect of identical global change manipulations on the introduced lineage of P. australis (haplotype M) and its encroachment into the native marsh community. For details of chamber setup and experimental design see Langley and Megonigal10 and Caplan et al.22. Briefly, half of the plots in each experiment are fertilized with NH4Cl at a rate of 25 g N m−2 yr−1 and half of the plots at each N level are fumigated with sufficient CO2 to raise the atmospheric concentration within open-top chambers by approximately 300 ppm throughout the growing season (May through November).
Aboveground biomass is estimated in both experiments in late July or early August of each year. For S. americanus and P. australis, this entails combining stem density counts with measurements of stem height and width that are converted to dry mass using species-specific allometric relationships77. For S. patens, biomass is measured directly by clipping samples within each chamber. Rhizome productivity is estimated from annual samples collected each year using ingrowth cores, with three placed in each plot for the native marsh study and six placed in each plot for the P. australis study.
Biomass data from the two long-term field experiments were used to quantify productivity responses to global change factors. Specifically, we calculated stimulation effects (i.e., differences from ambient) for the elevated CO2 treatment, the N enrichment treatment, and the combination treatment for both aboveground biomass and rhizome biomass. For the native marsh study, we used biomass data from S. americanus and S. patens spanning the first five years that data were available (2006–2010 for aboveground biomass and 2007–2011 for rhizome biomass). Biomass data for P. australis came from the second study, but likewise spanning the first five years of its lifespan (2011–2015).
Data availability
The datasets used in this study are available from the corresponding author on reasonable request.
Acknowledgements
This work was primarily supported by the Irish Research Council, Marie Curie Actions, and Bryn Mawr College. The field experiment was supported by grants from NSF LTREB (awards DEB-0950080 and DEB-1457100) and Maryland Sea Grant (award SA7528114-WW). Additional support was provided by the Smithsonian Environmental Research Center and a Bucher-Jackson Postdoctoral Fellowship to JSC. The authors would like to thank Patrick Megonigal, Marcel Jansen, Adam Langley, Gary Peresta, Andrew Peresta, Jim Duls, Esha Ray, and Laura Silla for their valuable contributions. The authors have no conflicts of interest with respect to this research.
Author Contributions
T.J.M. designed the experiment and J.S.C. lead its execution. G.M.C. and J.S.C. conducted the data analysis and J.S.C. prepared the figures. G.M.C. led manuscript preparation, with J.S.C. and T.J.M. providing substantial input.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Solomon, S. et al. The physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change, 235–337 (2007).
- 2.Luo Y. Terrestrial carbon–cycle feedback to climate warming. Annu. Rev. Ecol. Evol. Syst. 2007;38:683–712. doi: 10.1146/annurev.ecolsys.38.091206.095808. [DOI] [Google Scholar]
- 3.Mcleod E, et al. A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Frontiers in Ecology and the Environment. 2011;9:552–560. doi: 10.1890/110004. [DOI] [Google Scholar]
- 4.Berntson G, Rajakaruna N, Bazzaz F. Growth and nitrogen uptake in an experimental community of annuals exposed to elevated atmospheric CO2. Global Change Biology. 1998;4:607–626. doi: 10.1046/j.1365-2486.1998.00171.x. [DOI] [Google Scholar]
- 5.Oren R, et al. Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enriched atmosphere. Nature. 2001;411:469–472. doi: 10.1038/35078064. [DOI] [PubMed] [Google Scholar]
- 6.Hungate BA, Dukes JS, Shaw MR, Luo Y, Field CB. Nitrogen andclimate change. Science. 2003;302:1512–1513. doi: 10.1126/science.1091390. [DOI] [PubMed] [Google Scholar]
- 7.Chapin FS., III The mineral nutrition of wild plants. Annual review of ecology and systematics. 1980;11:233–260. doi: 10.1146/annurev.es.11.110180.001313. [DOI] [Google Scholar]
- 8.Forde BG, Clarkson DT. Nitrate and ammonium nutrition of plants: physiological and molecular perspectives. Advances in botanical research. 1999;30:1–90. doi: 10.1016/S0065-2296(08)60226-8. [DOI] [Google Scholar]
- 9.Bassirirad H. Kinetics of nutrient uptake by roots: responses to global change. New phytologist. 2000;147:155–169. doi: 10.1046/j.1469-8137.2000.00682.x. [DOI] [Google Scholar]
- 10.Langley JA, Megonigal JP. Ecosystem response to elevated CO2 levels limited by nitrogen-induced plant species shift. Nature. 2010;466:96–99. doi: 10.1038/nature09176. [DOI] [PubMed] [Google Scholar]
- 11.Bertness MD, Ewanchuk PJ, Silliman BR. Anthropogenic modification of New England salt marsh landscapes. Proceedings of the National Academy of Sciences. 2002;99:1395–1398. doi: 10.1073/pnas.022447299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King RS, Deluca WV, Whigham DF, Marra PP. Threshold effects of coastal urbanization onPhragmites australis (common reed) abundance and foliar nitrogen in Chesapeake Bay. Estuaries and Coasts. 2007;30:469–481. doi: 10.1007/BF02819393. [DOI] [Google Scholar]
- 13.Mozdzer TJ, Zieman JC, McGlathery KJ. Nitrogen uptake by native and invasive temperate coastal macrophytes: importance of dissolved organic nitrogen. Estuaries and Coasts. 2010;33:784–797. doi: 10.1007/s12237-009-9254-9. [DOI] [Google Scholar]
- 14.Mozdzer T, Kirwan M, McGlathery K, Zieman J. Nitrogen uptake by the shoots of smooth cordgrass Spartina alterniflora. Marine Ecology Progress Series. 2011;433:43–52. doi: 10.3354/meps09117. [DOI] [Google Scholar]
- 15.Cott GM, Chapman DV, Jansen MA. Differences in nitrogen‐assimilating enzyme activity in halophyte species are habitat‐related. Journal of Plant Nutrition and Soil Science. 2014;177:705–713. doi: 10.1002/jpln.201300191. [DOI] [Google Scholar]
- 16.Morris, J. T. & Dacey, J. W. Effects of O2 on ammonium uptake and root respiration by Spartina alterniflora. American Journal of Botany, 979–985 (1984).
- 17.Morris JT. Effects of oxygen and salinity on ammonium uptake by Spartina alterniflora Loisel. and Spartina patens (Aiton) Muhl. Journal of Experimental Marine Biology and Ecology. 1984;78:87–98. doi: 10.1016/0022-0981(84)90071-6. [DOI] [Google Scholar]
- 18.Bradley PM, Morris JT. Influence of oxygen and sulfide concentration on nitrogen uptake kinetics in Spartina alterniflora. Ecology. 1990;71:282–287. doi: 10.2307/1940267. [DOI] [Google Scholar]
- 19.Ainsworth EA, Long SP. What have we learned from 15 years of free‐air CO2 enrichment (FACE)? A meta‐analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist. 2005;165:351–372. doi: 10.1111/j.1469-8137.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- 20.Talhelm AF, et al. Elevated carbon dioxide and ozone alter productivity and ecosystem carbon content in northern temperate forests. Global change biology. 2014;20:2492–2504. doi: 10.1111/gcb.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drake BG. Rising sea level, temperature, and precipitation impact plant and ecosystem responses to elevated CO2 on a Chesapeake Bay wetland: review of a 28‐year study. Global change biology. 2014;20:3329–3343. doi: 10.1111/gcb.12631. [DOI] [PubMed] [Google Scholar]
- 22.Caplan JS, Hager RN, Megonigal JP, Mozdzer TJ. Global change accelerates carbon assimilation by a wetland ecosystem engineer. Environ Res Lett. 2015;10:115006. doi: 10.1088/1748-9326/10/11/115006. [DOI] [Google Scholar]
- 23.Erickson JE, Megonigal JP, Peresta G, Drake BG. Salinity and sea level mediate elevated CO2 effects on C3–C4 plant interactions and tissue nitrogen in a Chesapeake Bay tidal wetland. Global Change Biology. 2007;13:202–215. doi: 10.1111/j.1365-2486.2006.01285.x. [DOI] [Google Scholar]
- 24.Erickson JE, Peresta G, Montovan KJ, Drake BG. Direct and indirect effects of elevated atmospheric CO2 on net ecosystem production in a Chesapeake Bay tidal wetland. Global change biology. 2013;19:3368–3378. doi: 10.1111/gcb.12316. [DOI] [PubMed] [Google Scholar]
- 25.Taub DR, Wang X. Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. Journal of Integrative Plant Biology. 2008;50:1365–1374. doi: 10.1111/j.1744-7909.2008.00754.x. [DOI] [PubMed] [Google Scholar]
- 26.Wong S-C. Elevated atmospheric partial pressure of CO2 and plant growth: II. Non-structural carbohydrate content in cotton plants and its effect on growth parameters. Photosynthesis Research. 1990;23:171–180. doi: 10.1007/BF00035008. [DOI] [PubMed] [Google Scholar]
- 27.Kuehny JS, Peet MM, Nelson PV, Willits DH. Nutrient dilution by starch in CO2-enriched Chrysanthemum. Journal of Experimental Botany. 1991;42:711–716. doi: 10.1093/jxb/42.6.711. [DOI] [Google Scholar]
- 28.Gifford RM, Barrett DJ, Lutze JL. The effects of elevated [CO2] on the C: N and C: P mass ratios of plant tissues. Plant and Soil. 2000;224:1–14. doi: 10.1023/A:1004790612630. [DOI] [Google Scholar]
- 29.McDonald EP, Erickson JE, Kruger EL. Research note: Can decreased transpiration limit plant nitrogen acquisition in elevated CO2? Functional Plant Biology. 2002;29:1115–1120. doi: 10.1071/FP02007. [DOI] [PubMed] [Google Scholar]
- 30.Hocking P, Meyer C. Effects of CO2 enrichment and nitrogen stress on growth, and partitioning of dry matter and nitrogen in wheat and maize. Functional Plant Biology. 1991;18:339–356. [Google Scholar]
- 31.Kimball B, et al. Elevated CO2, drought and soil nitrogen effects on wheat grain quality. New Phytologist. 2001;150:295–303. doi: 10.1046/j.1469-8137.2001.00107.x. [DOI] [Google Scholar]
- 32.Bloom AJ, Burger M, Asensio JSR, Cousins AB. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science. 2010;328:899–903. doi: 10.1126/science.1186440. [DOI] [PubMed] [Google Scholar]
- 33.Zerihun A, Bassirirad H. Interspecies variation in nitrogen uptake kinetic responses of temperate forest species to elevated CO2: potential causes and consequences. Global Change Biology. 2001;7:211–222. doi: 10.1046/j.1365-2486.2001.00384.x. [DOI] [Google Scholar]
- 34.Bassirirad H, Griffin KL, Strain BR, Reynolds JF. Effects of CO2 enrichment on growth and root 15 NH4. Tree physiology. 1996;16:957–962. doi: 10.1093/treephys/16.11-12.957. [DOI] [PubMed] [Google Scholar]
- 35.BassiriRad H, Thomas R, Reynolds J, Strain B. Differential responses of root uptake kinetics of NH4+ and NO3− to enriched atmospheric CO2 concentration in field‐grown loblolly pine. Plant, Cell & Environment. 1996;19:367–371. doi: 10.1111/j.1365-3040.1996.tb00260.x. [DOI] [Google Scholar]
- 36.BassiriRad H, Griffin KL, Reynolds JF, Strain BR. Changes in root NH4+ and NO3− absorption rates of loblolly and ponderosa pine in response to CO2 enrichment. Plant and Soil. 1997;190:1–9. doi: 10.1023/A:1004206624311. [DOI] [Google Scholar]
- 37.BassiriRad H, Reynolds J, Virginia R, Brunelle M. Growth and root NO3-and PO43-uptake capacity of three desert species in response to atmospheric CO2 enrichment. Functional Plant Biology. 1997;24:353–358. [Google Scholar]
- 38.Newbery R, Wolfenden J, Mansfield T, Harrison A. Nitrogen, phosphorus and potassium uptake and demand in Agrostis capillaris: the influence of elevated CO2 and nutrient supply. New Phytologist. 1995;130:565–574. doi: 10.1111/j.1469-8137.1995.tb04333.x. [DOI] [PubMed] [Google Scholar]
- 39.Jackson R, Reynolds H. Nitrate and ammonium uptake for single-and mixed-species communities grown at elevated CO2. Oecologia. 1996;105:74–80. doi: 10.1007/BF00328793. [DOI] [PubMed] [Google Scholar]
- 40.Cruz C, Lips S, Martins-Louçzão M. Changes in the morphology of roots and leaves of carob seedlings induced by nitrogen source and atmospheric carbon dioxide. Annals of Botany. 1997;80:817–823. doi: 10.1006/anbo.1997.0524. [DOI] [Google Scholar]
- 41.Ow, Y. X. et al. Nitrate fertilisation does not enhance CO2 responses in two tropical seagrass species. Scientific Reports6, 23093; 10.1038/srep23093 (2016). [DOI] [PMC free article] [PubMed]
- 42.Igamberdiev AU, Bykova NV, Lea PJ, Gardeström P. The role of photorespiration in redox and energy balance of photosynthetic plant cells: a study with a barley mutant deficient in glycine decarboxylase. Physiologia Plantarum. 2001;111:427–438. doi: 10.1034/j.1399-3054.2001.1110402.x. [DOI] [PubMed] [Google Scholar]
- 43.Bloom AJ, et al. CO2 enrichment inhibits shoot nitrate assimilation in C3 but not C4 plants and slows growth under nitrate in C3 plants. Ecology. 2012;93:355–367. doi: 10.1890/11-0485.1. [DOI] [PubMed] [Google Scholar]
- 44.Kirwan ML, Megonigal JP. Tidal wetland stability in the face of human impacts and sea-level rise. Nature. 2013;504:53–60. doi: 10.1038/nature12856. [DOI] [PubMed] [Google Scholar]
- 45.Saltonstall K. Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proceedings of the National Academy of Sciences. 2002;99:2445–2449. doi: 10.1073/pnas.032477999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Craine, J. M. Resource strategies of wild plants. (Princeton University Press, 2009).
- 47.Tylova-Munzarova E, Lorenzen B, Brix H, Votrubova O. The effects of NH4+ and NO3− on growth, resource allocation and nitrogen uptake kinetics of Phragmites australis and Glyceria maxima. Aquatic Botany. 2005;81:326–342. doi: 10.1016/j.aquabot.2005.01.006. [DOI] [Google Scholar]
- 48.Chambers RM, Mozdzer TJ, Ambrose JC. Effects of salinity and sulfide on the distribution of Phragmites australis and Spartina alterniflora in a tidal saltmarsh. Aquatic Botany. 1998;62:161–169. doi: 10.1016/S0304-3770(98)00095-3. [DOI] [Google Scholar]
- 49.Romero JA, Brix H, Comín FA. Interactive effects of N and P on growth, nutrient allocation and NH 4 uptake kinetics by Phragmites australis. Aquatic Botany. 1999;64:369–380. doi: 10.1016/S0304-3770(99)00064-9. [DOI] [Google Scholar]
- 50.Mozdzer TJ, Megonigal JP. Jack-and-master trait responses to elevated CO2 and N: a comparison of native and introduced Phragmites australis. PLoS One. 2012;7:e42794. doi: 10.1371/journal.pone.0042794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kronzucker H, Glass A, Siddiqi M, Kirk G. Comparative kinetic analysis of ammonium and nitrate acquisition by tropical lowland rice: implications for rice cultivation and yield potential. New Phytologist. 2000;145:471–476. doi: 10.1046/j.1469-8137.2000.00606.x. [DOI] [PubMed] [Google Scholar]
- 52.Min X, Siddiqi MY, Guy RD, Glass AD, Kronzucker HJ. A comparative kinetic analysis of nitrate and ammonium influx in two early‐successional tree species of temperate and boreal forest ecosystems. Plant, Cell & Environment. 2000;23:321–328. doi: 10.1046/j.1365-3040.2000.00546.x. [DOI] [Google Scholar]
- 53.Maire V, Gross N, da Silveira Pontes L, Picon‐Cochard C, Soussana JF. Trade‐off between root nitrogen acquisition and shoot nitrogen utilization across 13 co‐occurring pasture grass species. Functional Ecology. 2009;23:668–679. doi: 10.1111/j.1365-2435.2009.01557.x. [DOI] [Google Scholar]
- 54.Mozdzer TJ, Langley JA, Mueller P, Megonigal JP. Deep rooting and global change facilitate spread of invasive grass. Biological Invasions. 2016;18:2619–2631. doi: 10.1007/s10530-016-1156-8. [DOI] [Google Scholar]
- 55.Nacry P, Bouguyon E, Gojon A. Nitrogen acquisition by roots: physiological and developmental mechanisms ensuring plant adaptation to a fluctuating resource. Plant and Soil. 2013;370:1–29. doi: 10.1007/s11104-013-1645-9. [DOI] [Google Scholar]
- 56.Clarkson D, Jones L, Purves J. Absorption of nitrate and ammonium ions by Lolium perenne from flowing solution cultures at low root temperatures. Plant, Cell & Environment. 1992;15:99–106. doi: 10.1111/j.1365-3040.1992.tb01462.x. [DOI] [Google Scholar]
- 57.Schenk M. Regulation of nitrogen uptake on the whole plant level. Plant and Soil. 1996;181:131–137. doi: 10.1007/BF00011299. [DOI] [Google Scholar]
- 58.Caplan, J. S. et al. Nutrient foraging strategies are associated with productivity and population growth in forest shrubs. Annals of Botany119, 977–988 (2017). [DOI] [PMC free article] [PubMed]
- 59.Grassein F, et al. Relationships between functional traits and inorganic nitrogen acquisition among eight contrasting European grass species. Annals of botany. 2015;115:107–115. doi: 10.1093/aob/mcu233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Curtis PS, Drake BG, Whigham DF. Nitrogen and carbon dynamics in C3 and C4 estuarine marsh plants grown under elevated CO2in situ. Oecologia. 1989;78:297–301. doi: 10.1007/BF00379101. [DOI] [PubMed] [Google Scholar]
- 61.Hymus GJ, Snead TG, Johnson DP, Hungate BA, Drake BG. Acclimation of photosynthesis and respiration to elevated atmospheric CO2 in two Scrub Oaks. Global Change Biology. 2002;8:317–328. doi: 10.1046/j.1354-1013.2001.00472.x. [DOI] [Google Scholar]
- 62.Bloom AJ, Sukrapanna SS, Warner RL. Root respiration associated with ammonium and nitrate absorption and assimilation by barley. Plant Physiology. 1992;99:1294–1301. doi: 10.1104/pp.99.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poorter H, Gifford RM, Kriedemann PE, Wong SC. A quantitative-analysis of dark respiration and carbon content as factors in the growth-response of plants to elevated CO2. Australian Journal of Botany. 1992;40:501–513. doi: 10.1071/BT9920501. [DOI] [Google Scholar]
- 64.Drake BG, et al. Does elevated atmospheric CO2 concentration inhibit mitochondrial respiration in green plants? Plant, Cell & Environment. 1999;22:649–657. doi: 10.1046/j.1365-3040.1999.00438.x. [DOI] [Google Scholar]
- 65.Smith NG, Dukes JS. Plant respiration and photosynthesis in global‐scale models: incorporating acclimation to temperature and CO2. Global Change Biology. 2013;19:45–63. doi: 10.1111/j.1365-2486.2012.02797.x. [DOI] [PubMed] [Google Scholar]
- 66.Rawat SR, Silim SN, Kronzucker HJ, Siddiqi MY, Glass AD. AtAMT1 gene expression and NH4+ uptake in roots of Arabidopsis thaliana: evidence for regulation by root glutamine levels. The Plant Journal. 1999;19:143–152. doi: 10.1046/j.1365-313X.1999.00505.x. [DOI] [PubMed] [Google Scholar]
- 67.Zhuo D, Okamoto M, Vidmar JJ, Glass AD. Regulation of a putative high‐affinity nitrate transporter (Nrt2; 1At) in roots ofArabidopsis thaliana. The Plant Journal. 1999;17:563–568. doi: 10.1046/j.1365-313X.1999.00396.x. [DOI] [PubMed] [Google Scholar]
- 68.Glass AD, et al. The regulation of nitrate and ammonium transport systems in plants. Journal of Experimental Botany. 2002;53:855–864. doi: 10.1093/jexbot/53.370.855. [DOI] [PubMed] [Google Scholar]
- 69.Oaks A, Hirel B. Nitrogen metabolism in roots. Annual Review of Plant Physiology. 1985;36:345–365. doi: 10.1146/annurev.pp.36.060185.002021. [DOI] [Google Scholar]
- 70.Good AG, Shrawat AK, Muench DG. Can less yield more? Is reducing nutrient input into the environment compatible with maintaining crop production? Trends in plant science. 2004;9:597–605. doi: 10.1016/j.tplants.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 71.Coruzzi G, Bush DR. Nitrogen and carbon nutrient and metabolite signaling in plants. Plant Physiology. 2001;125:61–64. doi: 10.1104/pp.125.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arp, W. J., Drake, B. G., Pockman, W. T., Curtis, P. S. & Whigham, D. F. Interactions between C3 and C4 salt marsh plant species during four years of exposure to elevated atmospheric CO2. Vegetatio104, 133–143 (1993).
- 73.Holm, L. G., Plucknett, D. L., Pancho, J. V. & Herberger, J. P. The world’s worst weeds: distribution and biology. (The University Press of Hawaii, Honolulu, 1977).
- 74.Epstein E, Schmid WE, Rains D. Significance and technique of short-term experiments on solute absorption by plant tissue. Plant and Cell Physiology. 1963;4:79–84. doi: 10.1093/oxfordjournals.pcp.a078989. [DOI] [Google Scholar]
- 75.Hauck RD BJ. Use of tracers for soil and fertilizer research. Advances in Agronomy. 1976;28:219–266. doi: 10.1016/S0065-2113(08)60556-8. [DOI] [Google Scholar]
- 76.Christiansen NH, Andersen FØ, Jensen HS. Phosphate uptake kinetics for four species of submerged freshwater macrophytes measured by a 33 P phosphate radioisotope technique. Aquatic Botany. 2016;128:58–67. doi: 10.1016/j.aquabot.2015.10.002. [DOI] [Google Scholar]
- 77.Lu M, et al. Allometry data and equations for coastal marsh plants. Ecology. 2016;97:3554–3554. doi: 10.1002/ecy.1600. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used in this study are available from the corresponding author on reasonable request.