Figure 2.
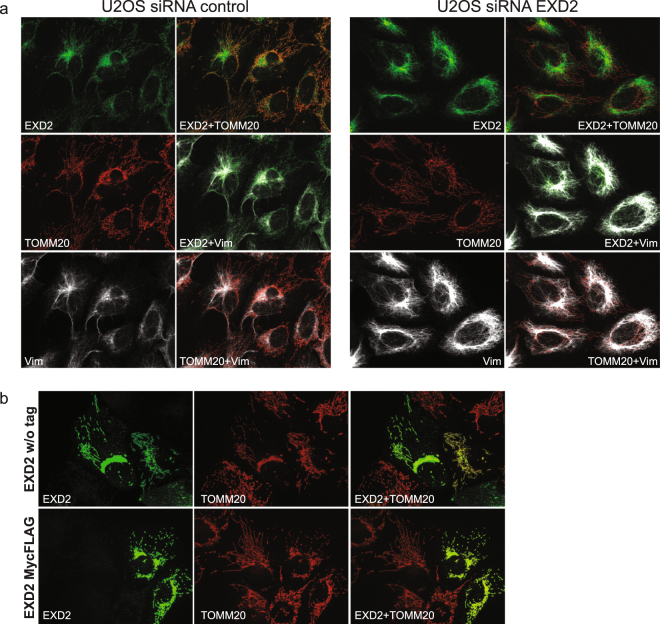
Knockdown or overexpression in U2OS cells of full-length EXD2 confirms the mitochondrial localization of EXD2. ProteinAtlas describes their EXD2 antibody, which we have used throughout this study, as having a mitochondrial and possible intermediate filament localization. To test the localization and the validity of their antibody we tested the EXD2 antibody, together with an antibody against the outer-membrane protein Tomm20 and an antibody against the intermediate filament protein vimentin (Vim) using immunofluorescence following transfection with either a pool of non-targeting control siRNAs or a pool of three EXD2 Stealth siRNAs (panel a). Co-staining in control siRNA cells with Tomm20 and vimentin shows co-localization of the EXD2 signal both with mitochondrial and intermediate filament signals. EXD2 siRNA treatment shows that while the EXD2 mitochondrial signal is no longer observed, the intermediate filament signal remains suggesting that this signal is either non-specific or that the siRNA pool used does not affect intermediate filament associated EXD2. Transient overexpression of the predicted full length protein, either w/o a tag or with a C-terminal combined Myc/FLAG tag shows an exclusive mitochondrial localization of the protein as illustrated by Tomm20 co-staining, while higher level overexpression results in mitochondrial perinuclear clustering (panel b). With very high overexpression, the whole mitochondrial network collapsed in one large perinuclear cluster that had lost any typical mitochondrial network-like structure (Supplementary Fig. S1). Overexpressed full length EXD2 showed no evidence of either nuclear or intermediate filament localization. Please note that for this Figure images have been selected deliberately to best illustrate the mitochondrial localization of EXD2. Image views have thus been chosen showing cells with a clear and extended mitochondrial network, while trying to avoid cells with a condensed/collapsed mitochondrial network.