Figure 3.
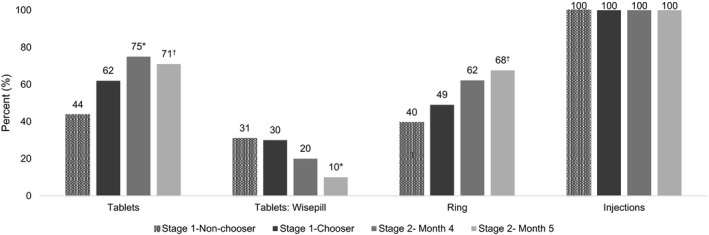
Product adherence during the TRIO study. Adherence was a multicomponent measure based on self‐reported use and direct observation of use during the study visits (initiation and return visit after 1 month of use). For injections, adherence was based on receiving two injections at the initiation visit by the study clinician. Adherence during stage 1 is shown for those who chose to use the product in stage 2 compared to those who did not chose to use the product in stage 2. Adherence improved during stage 2 for tablets (p = 0.04) and ring (p = 0.06). For tablets, objective use data was also available from Wisepill containers, which electronically track the opening of the tablet container (second set of bars from the left). Participants were considered adherent per Wisepill if the container was opened at least once per day for 80% of days during the month. For tablet use per Wisepill, there was a significant decrease in use over time (p = 0.002). *p < 0.05 †p < 0.10; p‐values from mixed‐effect logistic regression model.
