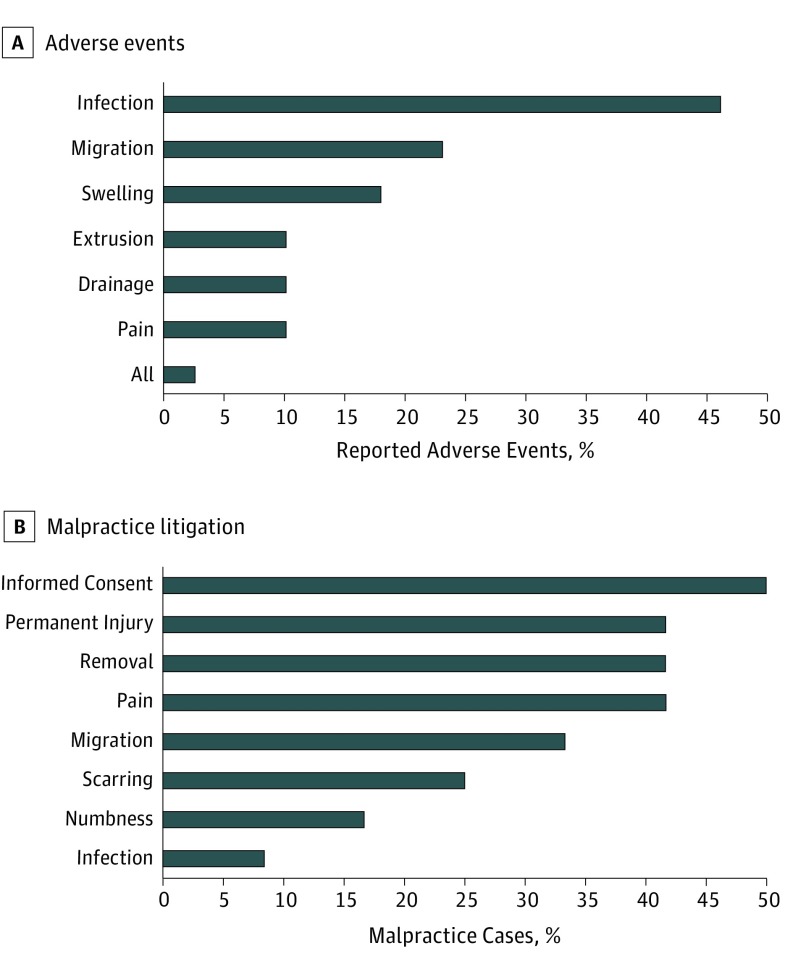
Figure 1. Complications Among Cases Reported to the US Food and Drug Administration and Allegations Raised in Relevant Litigation.
A, Infection was the most commonly reported adverse event, followed by migration, swelling, and extrusion. B, Allegedly inadequate informed consent was the issue most commonly cited in malpractice litigation. Permanent injury, removal of the implant, and pain were also commonly cited.