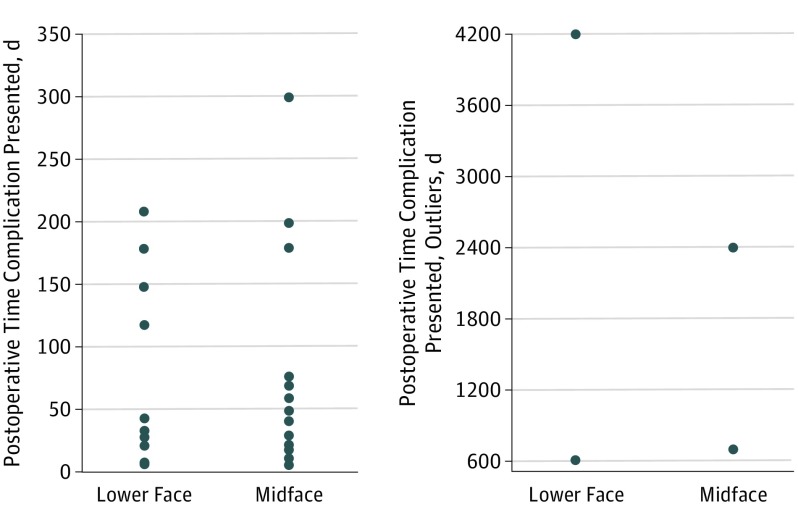
Figure 2. Timing of Adverse Events Among Complications Reported to the US Food and Drug Administration.
Reported events occurred during a wide range of times following implant placement. For lower face implants (ie, mandible and chin implants), these complications were reported at a median (range) of 45 (8-2400) days postoperatively compared with 45.5 (6-2400) days for midface implants.