Figure 3. Recording Ca2+ responses in living pancreatic slices reveals functional differences between mural cell populations.
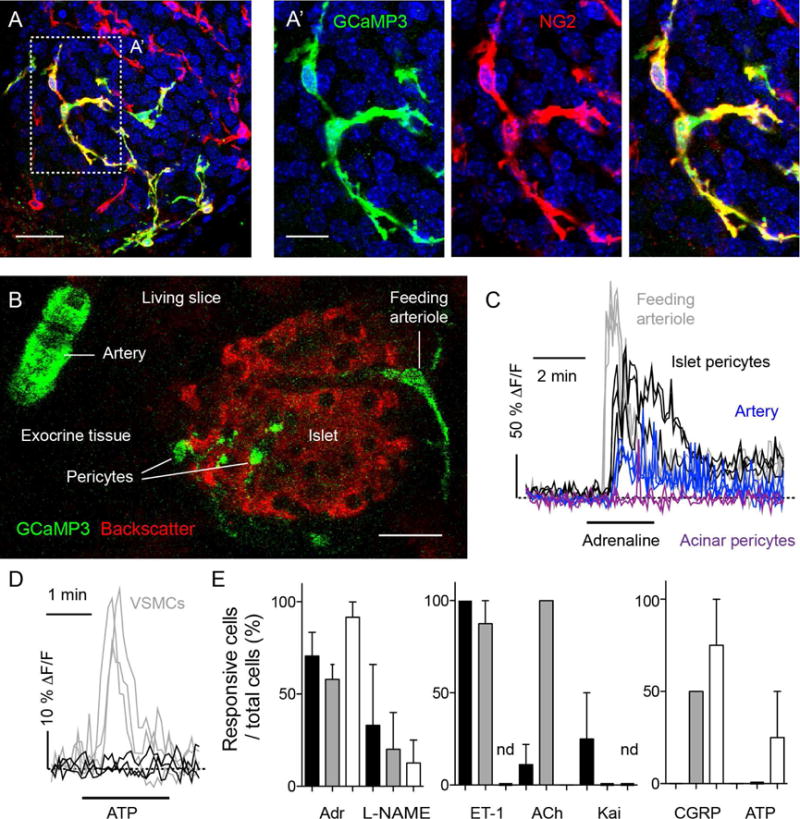
(A and A′) Z-stack of confocal images of a pancreatic slice from an NG2-GCaMP3 transgenic mouse processed for immunohistochemistry after a physiological experiment. Shown is an islet immunostained for GFP (GCaMP3, green) and NG2 (red). The Ca2+ sensor GCaMP3 is expressed in pericytes (colocalization appears yellow in merged images). Cell nuclei are shown in blue. Scale bars, 20 μm (A) and 10 μm (A′).
(B) Confocal image of a pancreatic slice from an NG2-GCaMP3 transgenic mouse showing mural cells expressing GCaMP3 (green) and islet endocrine cells (backscatter signal, red). GCaMP3 is expressed by different mural cells located around arteries, arterioles and within the islet parenchyma. Scale bar, 20 μm.
(C) Representative traces showing changes in mean GCaMP3 fluorescence intensity induced by adrenaline (100 μM) in islet pericytes (black), acinar pericytes (purple) and mural cells on the feeding arteriole (gray) or around the artery (blue). Increases in GCaMP3 fluorescence indicate increases in cytosolic Ca2+ levels and are expressed as ΔF/F. Dashed horizontal line indicates the zero value. Each trace corresponds to one cell.
(D) Representative traces showing changes in cytosolic Ca2+ levels in islet pericytes (black) and smooth muscle cells (VSMCs, gray) induced by ATP (100 μM). Dashed horizontal line indicates the zero value.
(E) Quantification of the percentage of islet pericytes (black bars), acinar pericytes (white bars) and smooth muscle cells (gray bars) that respond to different vasoactive substances: adrenaline (Adr, 50–100 μM), l-nitro-arginine methyl ester (L-NAME, 10 μM), endothelin-1 (ET-1, 10 nM), acetylcholine (ACh, 100 μM), kainate (Kai, 10 μM), calcitonin gene-related peptide (CGRP, 10 μM) and ATP (100 μM). (nd, not determined; N = 3 – 22 cells were examined per substance).
