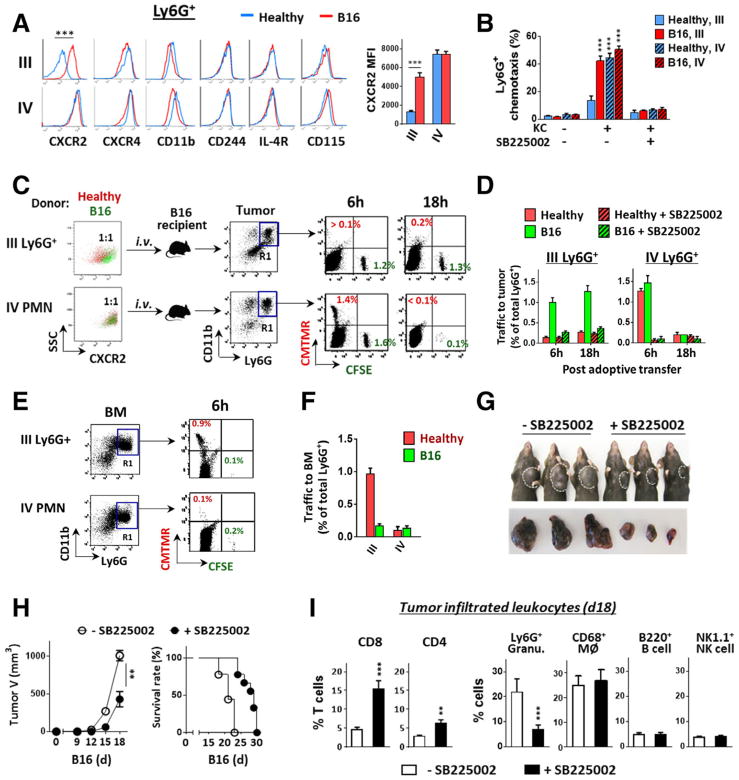
Figure 4.
Increased CXCR2 expression on G-MDSCs directs egress out of bone marrow into tumors. (A) Isolated bone marrow band III and IV Ly6G+ granulocytes from healthy and melanoma-bearing mice were detected for cell surface markers by flow cytometry. (B) G-MDSC and PMN chemotaxis toward CXCL1 (KC). In vitro chemotaxis assays were performed using transwell setups toward KC in the presence or absence of a CXCR2 antagonist SB225002). (C–F) In vivo granulocyte trafficking to B16 melanoma. Isolated band III Ly6G+ granulocytes and band IV mature PMN were labeled with fluorescence dye, CMTMR (red) for those originated from healthy mice, or CFSE (green) for those originated from melanoma-bearing mice. Mixed red and green (1:1 ratio) granulocytes of band III or IV were then transferred i.v. into melanoma-bearing recipient mice (tumor size ~ 500 mm3), without or along with SB225002. At 6 and 18 h post transfer, melanoma tumors (C and D) and femur bones (E–F) were excised and analyzed for colored granulocytes within CD11b+Ly6G+ cells (R1 gating), indicating donor Ly6G+ granulocyte trafficking. G–I). Mice engrafted with B16 were given (i.p., 3×) SB225002 or vehicle every other day. The effects on tumor growth (G and H), the overall survival rates (H), and the intratumoral T cells and other leukocytes (I) were determined on day 18. Flow data in left panel A, C and E are from a single experiment representative of at least three independent experiments with five mice per experiment. Data in A, B, D, F, H and I are presented as mean ± SEM and represent at least three experiments with more than five mice per experiment. The significant differences between the tested groups were calculated by two-tailed paired Student’s t-test (A, H, I) or one-way ANOVA followed by Dunnett’s Multiple Comparison test (B). **p ≤ 0.01; ***p ≤ 0.001.