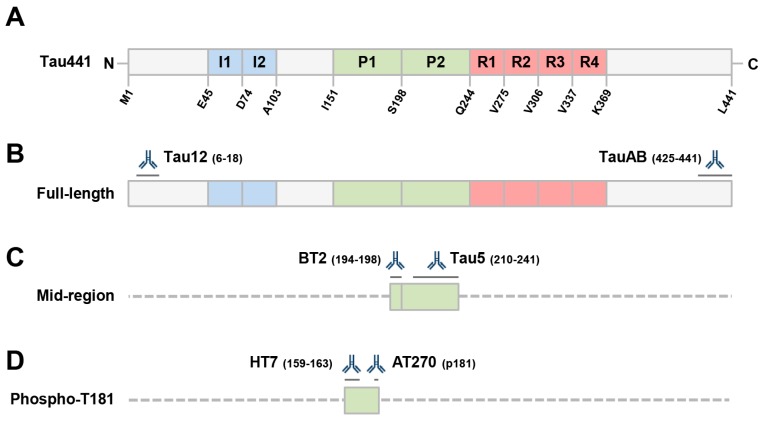
Figure 1.
Schematic depicting the longest isoform of human tau (441), the antibodies employed and the tau fragment(s) recognized by immunoassays used in this study. (A) Domain structure of tau, including the 2 N-terminal inserts (I1 and I2), the proline-rich domain (P1 and P2), and the microtube-binding repeat domain (R1 to R4), which is required for tau aggregation. (B) Both our full-length ELISA and Simoa assays employ Tau12, a mAb to the extreme N-terminus of tau, and TauAB, a mAb to the extreme C-terminus. (C) Our mid-region assay employs mAbs BT2 and Tau5 which recognize epitopes in the proline-rich domain. This assay is highly similar to those used in clinical research. (D) The Innotest phospho-tau kit (from Fujirebio) employs 2 mid-region directed mAbs; HT7, which recognizes an epitope spanning residues 159–163, and AT270, a mAb specific for phosphorylated threonine 181. Epitopes for the antibodies are indicated by dark grey lines. Sequences of minimal predicted length recognized by a given ELISA are shown in filled boxes and other potentially detectable forms of tau are indicated with dashed lines.