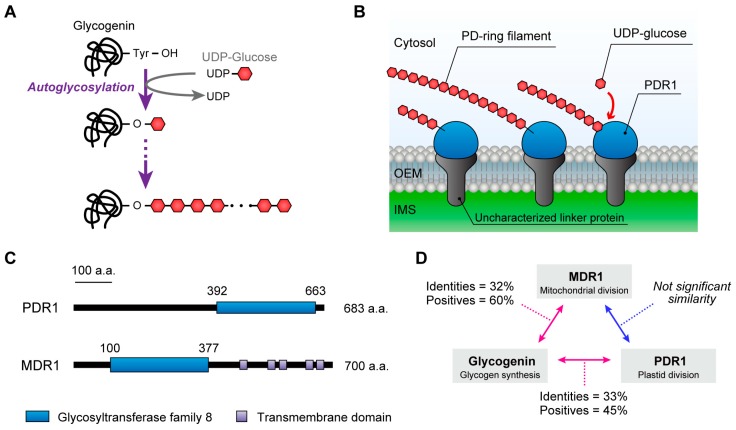
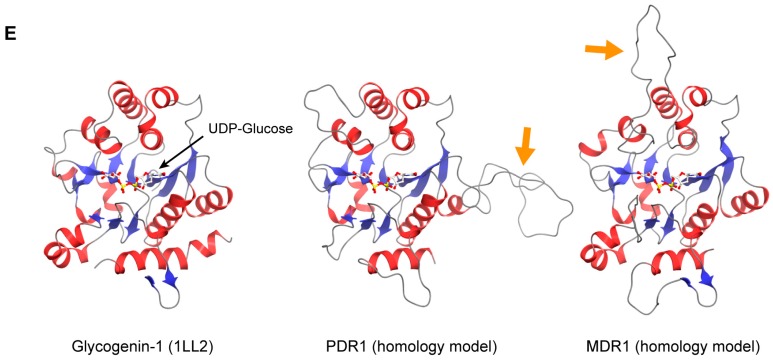
Figure 4.
Working models of the glycosyltransferases glycogenin and PDR1. (A) Glycogenin is required for the initiation of glycogen biosynthesis, and can be autoglycosylated at a specific tyrosine residue to form a short oligosaccharide chain of glucose molecules to act as a priming chain for the subsequent biosynthesis of glycogen. (B) A schematic representation of PD ring filament biosynthesis by PDR1. A series of results suggested that PD ring filaments are composed of both PDR1 and glucose molecules. Considering the sequence similarity with glycogenin, the glycosyltransferase domain of PDR1 may biosynthesize the polyglucan nanofilaments from UDP-glucose residues to form the PD ring filaments. OEM, outer envelope membrane; IMS, intermembrane space. (C) Schematic of C. merolae PDR1 and MDR1 domain structures. The glycosyltransferase domains of PDR1 and MDR1 identified them as type-8 subgroup members of the glycosyltransferase family. (D) Protein sequence similarities between PDR1, MDR1 and glycogenin-1. (E) Comparisons of the protein structure of glycogenin-1 (PDB: 1LL2) and the putative structures of the glycosyltransferase domains of PDR1 and MDR1. Orange arrows indicate specific insertion regions in the glycosyltransferase domains of PDR1 and MDR1. The protein structures of the PDR1 and MDR1 glycosyltransferase domains were modeled as described in Figure 2.