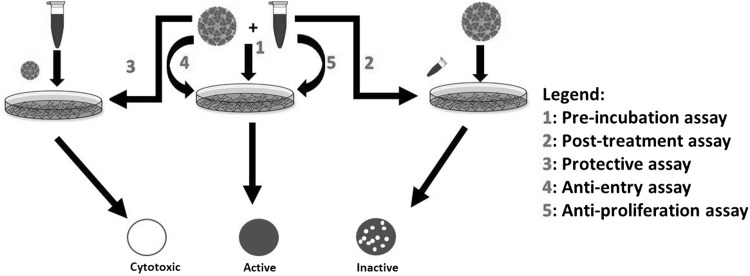
Fig. 1.
Schematic representation of in vitro bioassays developed for the understand of antiviral effect of the polyherbal formulations. 5 types of anti-viral assays were developed to evaluate the anti-viral activity of siddha formulations. All the assays were based on modified plaque reduction neutralization assay. Type 1 assay: Pre-incubation of CHIKV and siddha formulations at 4 °C overnight followed by PRNT assay with Vero cells. Type 2 assay: Post treatment of the CHIKV infected cells with siddha formulations at 37 °C followed by PRNT assays to evaluate the role of siddha formulations. Type 3 assay: to evaluate protective effect of polyherbal siddha formulations preceding CHIKV infection. Type4 assay: Anti-entry assay of CHIKV in the presence of siddha formulations at 4 °C followed by PRNT assay with Vero cells. Type 5 assay: Anti-proliferative assay of CHIKV in the presence of siddha formulations at 37 °C followed by PRNT assay with Vero cells