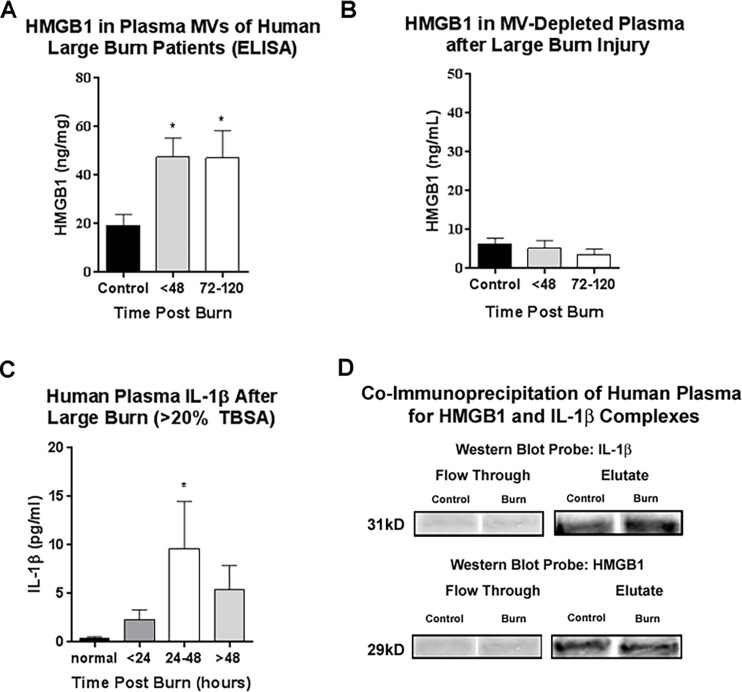
Fig 4. Human large burn injuries cause increased HMGB1 release in microvesicles (MVs), IL-β, and HMGB1/IL-1 complexes in plasma.
In two different patient groups of adult patients admitted to the North Carolina Jaycee Burn Center at the University of North Carolina at Chapel Hill hospital were enrolled into the study and serial blood draws were taken. IL-1β and HMGB1 were measured in plasma and plasma MVs respectively. (A-B) Plasma was collected from 15 human burn patients with a mean age of 36.5 (range 12–77); 80% male, mean %TBSA 14.5% (range 1.5–42%). Plasma collections were obtained during the first 48 hours of admission and between 72–120 hours after admission. See Table 2 for details. (A) Microvesicles (MVs) were isolated by centrifugation. HMGB1 was measured in MVs by ELISA. HMGB1 was increased in plasma MVs 2.5-fold after burn injury. This was observed both within 48 hours (47.6±7.73 vs. 19.32±4.5 ng/mg total protein, Burn vs Control) and at 72–120 hours after admission (47.09±19.5 vs. 19.32±4.5 ng/mg total protein, Burn vs Control, mean±SEM, *p<0.05) N = 3–6 per group. (B) HMGB1 was measured in MV-depleted plasma by ELISA. HMGB1 in MV-depleted plasma was unchanged after burn injury. (C-D) Plasma was collected from 22 patients, mean age 42 (range 18–78); 82% male, mean %TBSA 26% (range 5–45%), see Table 3. The first blood sample was obtained at 0–24 hours following initial burn injury, the second was obtained at 24–48 hours following injury, and the third was obtained at 48–72 hours following injury. (C) Large burn (>20% TBSA, n = 8) was associated with a significant (*p < 0.05) increase in IL-1β plasma concentration at 24–48 hours compared to control, normal individuals. (D) Co-immunoprecipitation was performed in human plasma to confirm the presence of HMGB1/IL-1β complexes. Western blot was performed and the flow through and eluate probed for IL-1β and HMGB1. Both the 31kD pro-form of IL-1β and HMGB1 were detected in the eluate, but not the flow through demonstrating the presence of HMGB1/pro-IL-1β complexes in human plasma.