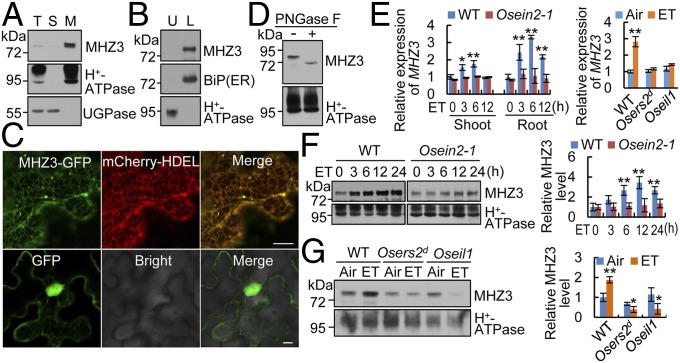
Fig. 2.
MHZ3 is an ER-localized glycosylated membrane protein, and its expression is induced by ethylene. (A) Membrane association of MHZ3. Equal amounts of total protein (T), soluble protein (S), and microsomal membranes (M) were immunoblotted for MHZ3, H+-ATPase (plasma membrane marker), and UGPase (cytoplasm marker). (B) Endomembrane association of MHZ3. Microsomal membranes were separated into plasma membrane (upper phase; U) and endomembrane (lower phase; L) systems and then immunoblotted for MHZ3, BiP, and H+-ATPase. (C) ER localization of MHZ3 as revealed by transient expression of MHZ3-GFP in tobacco leaf epidermal cells. mCherry-HDEL is used as an ER marker. GFP is used as a control. (Scale bars, 10 µm.) (D) Deglycosylation assay of MHZ3 using PNGase F. (E) MHZ3 transcript level is induced by 10 ppm ethylene (ET) as revealed by qRT-PCR analysis. Data are means ± SD, n = 3 [*P < 0.05, **P < 0.01; Student’s t test; compared with 0 h (Left) or Air (Right)]. (F and G) MHZ3 protein level is induced by ethylene, but the induction is impaired in ethylene-insensitive mutants. (Left) Membrane proteins isolated from rice shoots (F) or roots (G) of etiolated seedlings were immunoblotted for MHZ3 and H+-ATPase (loading control). (Right) Statistical analysis of the relative MHZ3 levels from three independent replicates is presented, and the data are means ± SD, n = 3 [*P < 0.05, **P < 0.01; Student’s t test; compared with 0 h (F) or Air (G)].