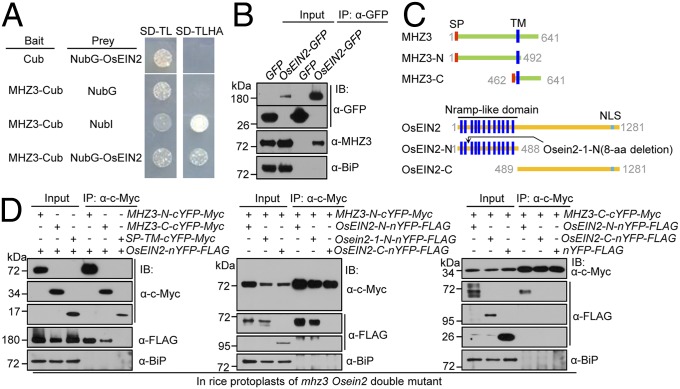
Fig. 4.
MHZ3 physically interacts with OsEIN2 through association with its N-terminal Nramp-like domain. (A) Split-ubiquitin Y2H assay for interaction of MHZ3 and OsEIN2. NubI is the WT N-terminal half of ubiquitin and serves as positive control. (B) Co-IP of MHZ3 with OsEIN2 in planta. Transgenic rice seedlings stably expressing 35S:OsEIN2-GFP or 35S:GFP were treated with 10 ppm ethylene for 3 d. Total proteins were immunoprecipitated with GFP-Trap and immunoblotted with anti-GFP, anti-MHZ3, and anti-BiP (internal control) antibodies. (C) Diagrams of full-length and truncated versions of MHZ3 (Upper) and OsEIN2 (Lower) used in interaction domain mapping studies. (D) Co-IP assays for interaction domain mapping of MHZ3 and OsEIN2. The SP-TM-cYFP-Myc construct contains SP and TM of MHZ3 and was used as a negative control. The constructs were cotransformed into rice protoplasts. Total proteins were immunoprecipitated with anti-Myc affinity gel and immunoblotted with anti-c-Myc, anti-FLAG, and anti-BiP antibodies.