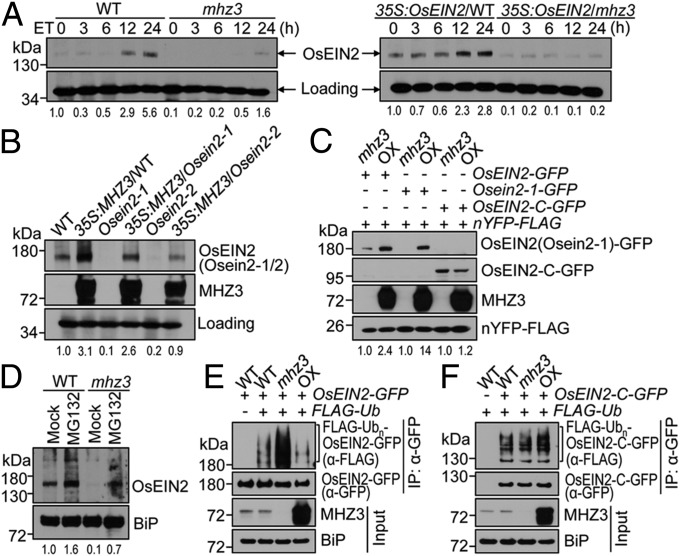
Fig. 5.
MHZ3 is required for OsEIN2 accumulation and impedes the protein ubiquitination. (A) Ethylene-induced OsEIN2 accumulation is impaired by mhz3 mutation. Etiolated 2-d-old seedlings of WT, mhz3, and 35S:OsEIN2 transgenic lines (the same lines as in Fig. 3B) were treated with 10 ppm ethylene for 0 to 24 h. Total proteins were immunoblotted for OsEIN2. A nonspecific band was used as a loading control. The values at the bottom indicate averages of relative OsEIN2 levels from three independent replicates; this is same in B–D. (B) MHZ3 overexpression elevates OsEIN2 abundance. Total proteins isolated from etiolated 2-d-old seedlings of WT, Osein2 mutants, and 35S:MHZ3 transgenic lines (the same lines as in Fig. 3 C and D) were immunoblotted for OsEIN2 and MHZ3. Others are as in A. (C) The Nramp-like domain of OsEIN2 is crucial for MHZ3-mediated OsEIN2 accumulation. The constructs were cotransformed into mhz3 and MHZ3-OX22 (OX) protoplasts. nYFP-FLAG served as an internal control for normalizing the transformation efficiency. Total proteins were immunoblotted with anti-GFP, anti-FLAG, and anti-MHZ3 antibodies. (D) OsEIN2 protein in mhz3 mutant is stabilized by MG132. (E and F) Ubiquitination analysis for OsEIN2 (E) and OsEIN2-C (F) in different MHZ3 backgrounds. The constructs were cotransformed into rice protoplasts and incubated in the presence of 3 µM MG132 for 16 h. Total proteins were immunoprecipitated using GFP-Trap and immunoblotted with anti-FLAG and anti-GFP antibodies. Input proteins were immunoblotted for MHZ3 and BiP (loading control).