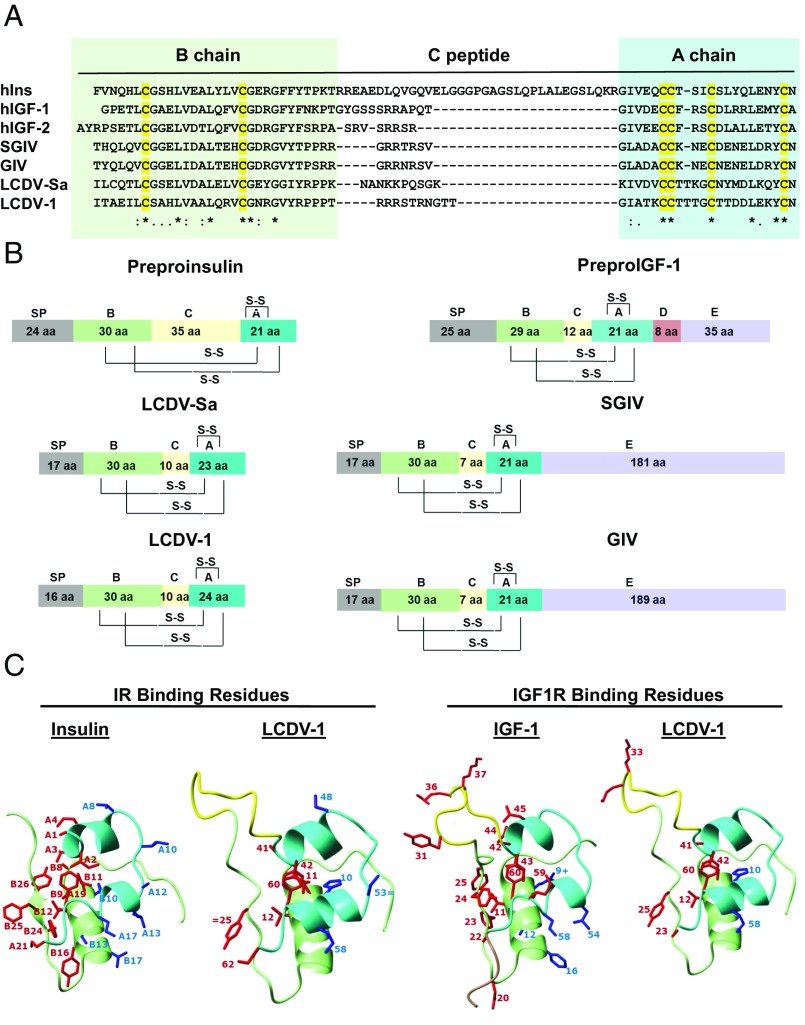
Fig. 1.
Viral insulin/IGF-like peptides are structurally a part of the insulin superfamily. (A) Sequence alignment of the B-, C-, and A-chains of insulin, IGF1, IGF2, and four VILPs. Cysteines are highlighted in yellow. Identical residues are denoted by asterisks; low and high degrees of similarity are represented by a period and a colon, respectively. (B) Domain structure of human insulin, IGF1, and four VILPs. The domains are indicated as follows: A, A-chain; B, B-chain; C, C-peptide; S-S, disulfide bonds; and SP, signal peptide. (C) Predicted 3D structure of LCDV-1VILP and comparison with insulin and IGF-I. The A-chain is cyan; the B-chain is light green; the C-peptide is yellow; and the D-domain is pale brown. The conserved or conservatively substituted side chains of residues of the ligands involved in binding to site 1 of the IR/IGF1R are shown in red, and binding site 2 residues are shown in blue. Conservative substitutions are indicated by an equal sign. One substitution that increases affinity in IGF-I (B10 His to Glu) is indicated by a plus sign.