Fig. 3.
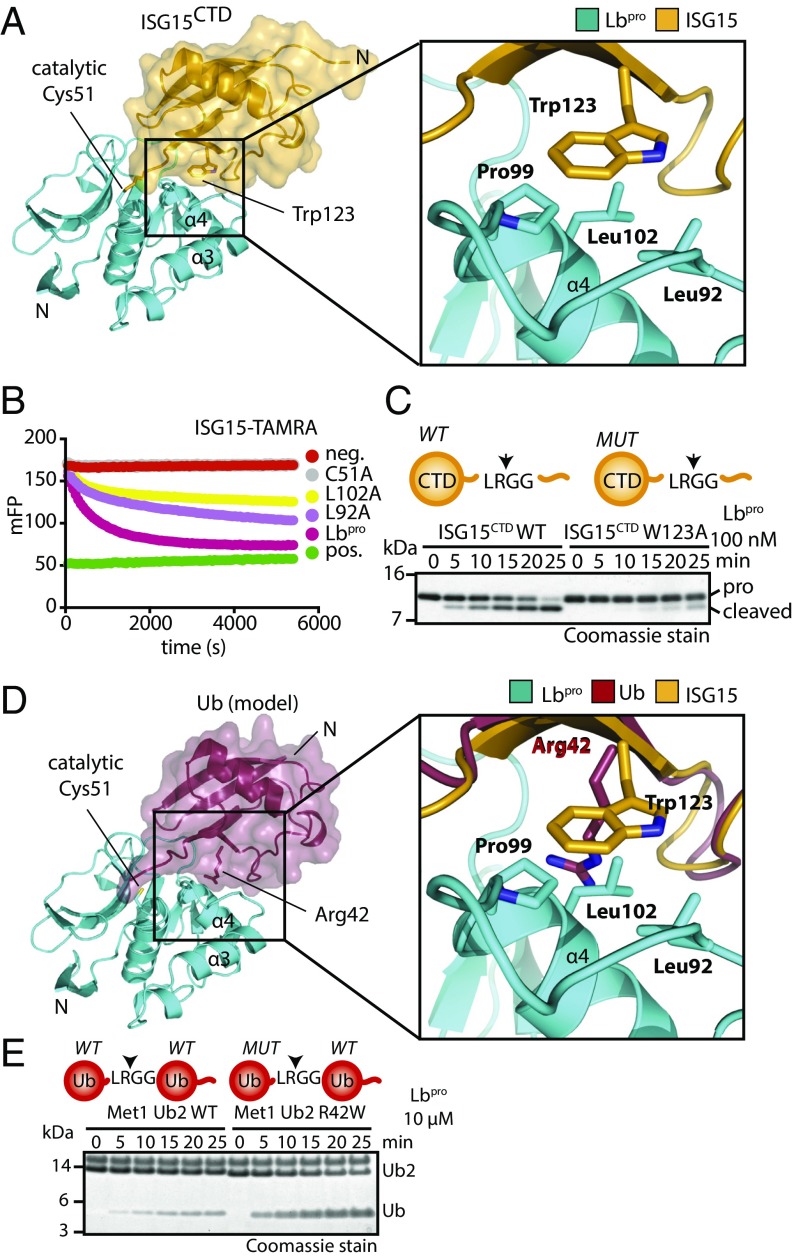
Structural analysis of ISG15 specificity. (A, Left) The Lbpro∼ISG15CTD-ΔC complex is shown as in Fig. 2D. (A, Right) Close-up view of the hydrophobic contact between Lbpro (Leu92, Pro99, Leu102) and ISG15 (Trp123) (Fig. S4). (B) ISG15-TAMRA cleavage assays with Lbpro point mutants in the hydrophobic patch. (C) Lbpro cleavage assays with WT and W123A proISG15CTD. (D, Left) Model of ubiquitin (Protein Data Bank ID code 1ubq) bound to Lbpro. Ubiquitin (red) was superimposed onto ISG15CTD. (D, Right) Close-up view of the analogous interface as in A. (E) Met1 diubiquitin cleavage assays with or without R42W mutation in the distal ubiquitin. Arrows in the schematics show the proteolytic site of cleavage (Fig. S4E). Assays in B, C, and E were performed in triplicate.