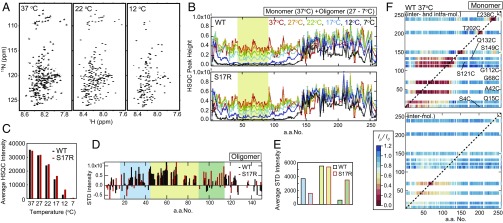
Fig. 3.
Sup35NM monomers form latent compact local structures with long-range interactions. (A) Temperature-dependent changes of 1H-15N HSQC spectra of WT Sup35NM. (B) Signal intensities for 1H-15N cross-peaks in an HSQC spectrum of WT and S17R Sup35NM at 37 °C (red), 27 °C (orange), 22 °C (green), 17 °C (cyan), 12 °C (blue), and 7 °C (black). The yellow area indicates the amino acid region with significantly reduced signal intensities at low temperature, indicating the oligomer core region. (C) Temperature-dependent change of average 1H-15N HSQC signals in the yellow area in B (amino acids 45–90). (D) STD signal intensity of the N domain of WT (black) and S17R Sup35NM (red) at 22 °C. The blue and green areas indicate the amino acid regions with increased STD signals specific to WT and S17R Sup35NM, respectively. (E) Averaged STD signal intensities in the blue (amino acids 15–44), yellow (amino acids 45–90), and green (amino acids 91–115) areas in D are indicated by blue, yellow, and green bars, respectively. (F) Paramagnetic relaxation enhancement of WT Sup35NM monitored by 1H-15N HSQC signal intensity. A MTSL spin probe was introduced into each selected single-cysteine Sup35NM mutant (S4C, Q15C, A42C, G68C, G112C, S121C, Q132C, S149C, T202C, and L238C). Inter/intramolecular (Upper) and intermolecular (Lower) PRE values at 37 °C are shown as a color scale showing the ratio of the HSQC intensity of PRE measurement to the HSQC intensity of control measurement (Ip/I0 ratio). The red color of high PRE values indicates the presence of interactions between a MTSL spin probe and an amino acid residue of interest.