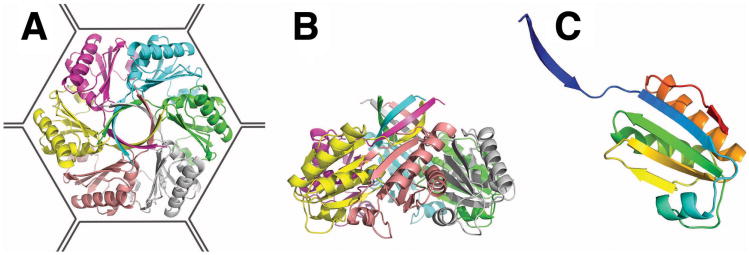
Figure 2.
Crystal structure of the PduU shell protein. (a) The PduU hexamer viewed along the sixfold axis and (b) perpendicular to the sixfold axis with distinct protein chains colored separately. The outline drawn around the hexamer (a) illustrates its presumed packing in the microcompartment shell amongst the other (more abundant) homologous shell protein hexamers (e.g. PduA, PduB, PduB', and PduJ). A prominent feature of the PduU hexamer is the six-stranded, parallel β-barrel formed by one N-terminal β-strand from each monomer. Whether this feature at the top of the hexamer faces out towards the cytosol or towards the interior of the microcompartment has not been established. (c) Ribbon diagram depicting the PduU monomer, colored from blue (N-terminal) to red (C-terminal). Over residue positions 19 to 109, the chain adopts a variation of the typical bacterial microcompartment (BMC) fold (Kerfeld et al., 2005). The eighteen amino-terminal, and eight carboxy-terminal residues form novel secondary structural elements.