Figure 6.
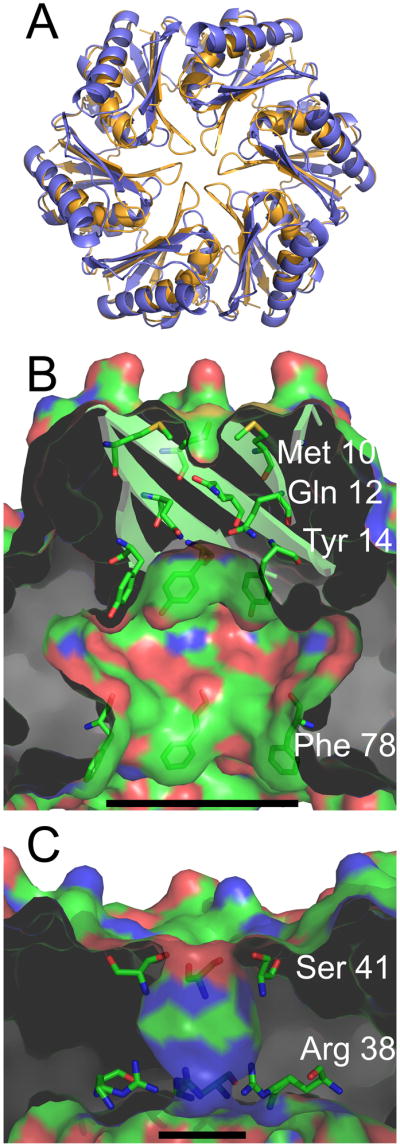
Properties of the central cavity in the PduU hexamer. (a) Comparison of a carboxysome shell protein hexamer, CcmK4 (orange) (Kerfeld et al., 2005), with the PduU hexamer (blue). To illustrate differences at the PduU central pore region, the N-termini are truncated so that only residues 19-116 are shown. (b) A cut-away view of the central cavity of the PduU hexamer, which is suggested here to be oriented with the open cavity facing the lumen of the Pdu microcompartment. The image is colored by atom type, with carbon (green), nitrogen (blue), and oxygen (red). Side chains from residues Met10, Gln12, Tyr14, and Phe78 are shown in order to illustrate their contributions to the surface. The substantial surface contributions by aromatic side chains are a distinction from carboxysome shell proteins whose pores are lined mainly by charged and polar side chains (Kerfeld et al., 2005; Yeates et al., 2007); CcmK4 is shown in panel (c). Measurement bars are added for scale, representing approximately 17 Å (b) and 8 Å (c). They indicate the distance between the Cβ atoms of Phe78 from opposing protein chains in panel (b) and between Arg38 N3 side chain atoms from opposing chains in panel (c) (17.1 Å and 7.8 Å, respectively). Note that the pore that is present in the carboxysome shell protein hexamer (panel c) is blocked by the β-barrel in PduU (panel b).
