Figure 2. Removal of CARD decreases caspase-9 activity.
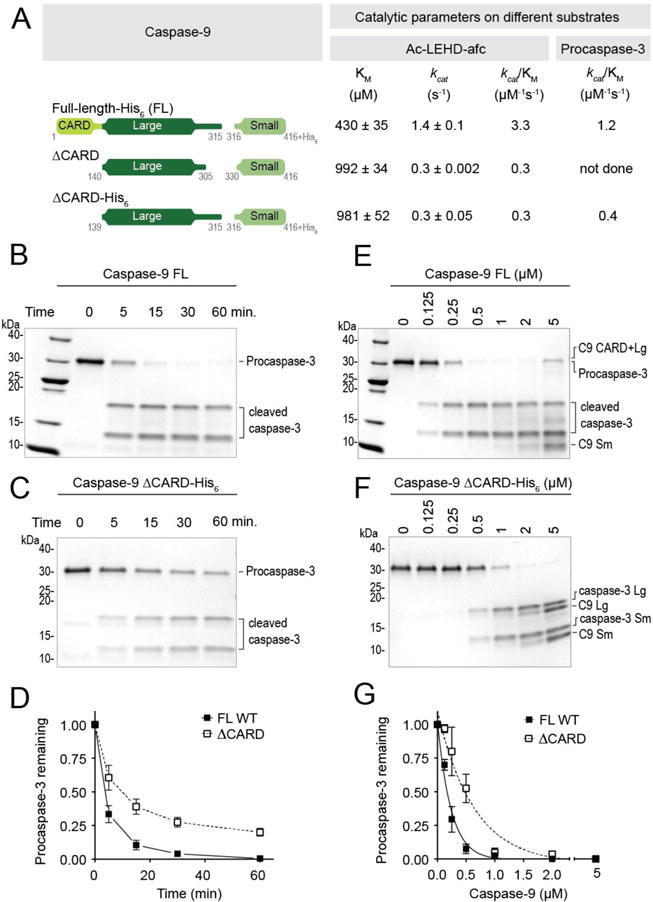
(A) Catalytic parameters of different versions of caspase-9 using Ac-LEHD-afc as a peptide substrate and procaspase-3 (C163S) as protein substrate. Two versions of ΔCARD were used in this study. Both versions exhibited very similar catalytic efficiencies. Using both substrates, ΔCARD was observed to be less active than full-length, wild-type (WT) caspase-9. Values reported are mean (+SEM) of three independent trials done on three separate days.
(B) and (C) ΔCARD has slower protein cleavage kinetics than full-length WT caspase-9. Catalytic cysteine mutant (C163S) procaspase-3 (3 μM) was incubated with 500 nM of either full-length, WT caspase-9 (B) or ΔCARD (C) for 1h at 37°C.
(D) Plot for time-course analysis of protein cleavage kinetics done on (B) and (C).
(E) and (F) Cleavage of procaspase-3 (C163S) to estimate kcat/KM of full-length WT caspase-9 and ΔCARD. 2 μM of procaspase-3 was incubated with either full-length WT (E) or ΔCARD (F) caspase-9 of different dilutions (0 - 5000 nM) for 1h at 37°C.
(G) Corresponding curves of (E) and (F) generated from the amount of procaspase-3 remaining and their non-linear fits to estimate kcat/KM.
