Figure 4. Monomeric and dimeric states of caspase-9 have different unfolding properties.
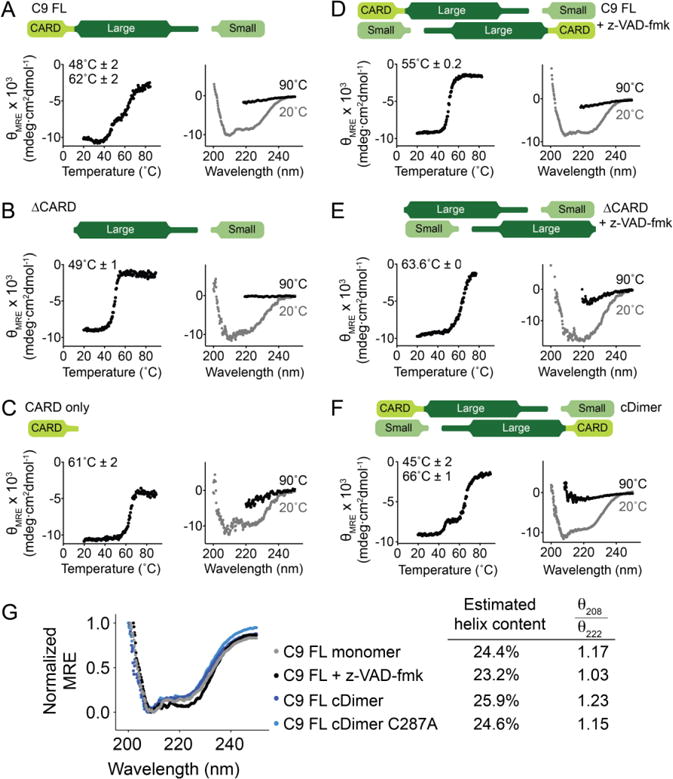
Thermal denaturation profile monitored by circular dichroism (left) with the thermal melting temperatures (Tm) for each transition listed and circular dichroism spectra (right) of various forms of caspase-9 (top schematics).
(A) Two melting transitions are observed in the cleaved full-length, monomeric, caspase-9.
(B) and (C) Thermal denaturation profile of cleaved caspase-9 core and the CARD, respectively, showing that the first melting transition in full-length, monomeric caspase-9 (A) is due to the unfolding of the core, while the second is due to that of the CARD.
(D) Full-length, cleaved caspase-9 is dimeric in the presence of an active-site ligand z-VAD-fmk. Upon thermal denaturation, caspase-9 in this state exhibits a single melting transition, likely due either to dimerization, or the presence of an ordered active site, or both.
(E) Dimeric, cleaved caspase-9 ΔCARD with bound z-VAD-fmk is stabilized by 14°C compared to monomeric, cleaved ΔCARD (B).
(F) Constitutive dimer (cDimer) full-length caspase-9 cleaved at the intersubunit linker exhibits two melting transitions, suggesting independent unfolding of CARD and core domains.
(G) The secondary structure of caspase-9 with (+ z-VAD-fmk) and without (FL monomer, cDimer and cDimer C287A) an active-site ligand bound was assessed by circular dichroism spectroscopy. C9 FL cDimer is a constitutive dimer variant of caspase-9. There is no significant change in the helical content of caspase-9 upon dimerization and substrate binding. Estimation of helix content was performed using BeStSel structure prediction and fold recognition software (http://bestsel.elte.hu/index.php) (62).
Plots are representative of three trials. Tm values shown are means ± SEM of three trials done on three separated days.
