Figure 5. The CARD and core of caspase-9 unfold as a single unit when the intersubunit linker is intact.
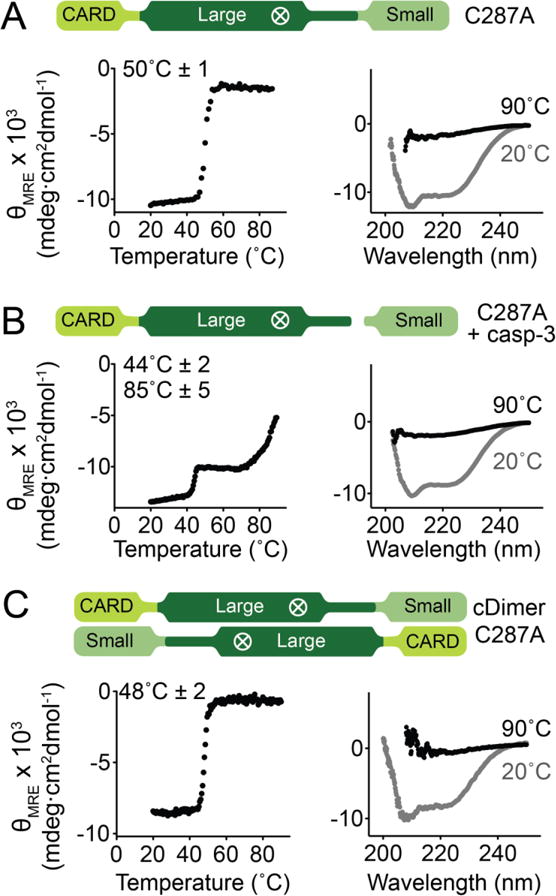
Thermal denaturation profiles monitored by circular dichroism (left) with the thermal melting temperatures (Tm) for each transition listed and circular dichroism spectra (right) of various forms of caspase-9.
(A) Monomeric, zymogen (uncleaved) caspase-9 (catalytic site-inactivated C287A) exhibited a single melting transition, which suggests cooperative unfolding of CARD and core of caspase-9.
(B) Cleavage of the linker of (A) by caspase-3 results in independent unfolding, as manifested by two separate melting transitions.
(C) Zymogen (catalytic site-inactivated C287A), uncleaved caspase-9 in a constitutively dimeric state (cDimer) shows a single melting transition.
Plots are representative of three trials. Tm values shown are means ± SEM of three trials done on three separated days.
