Figure 6. Linker between CARD and core supports CARD-core interaction.
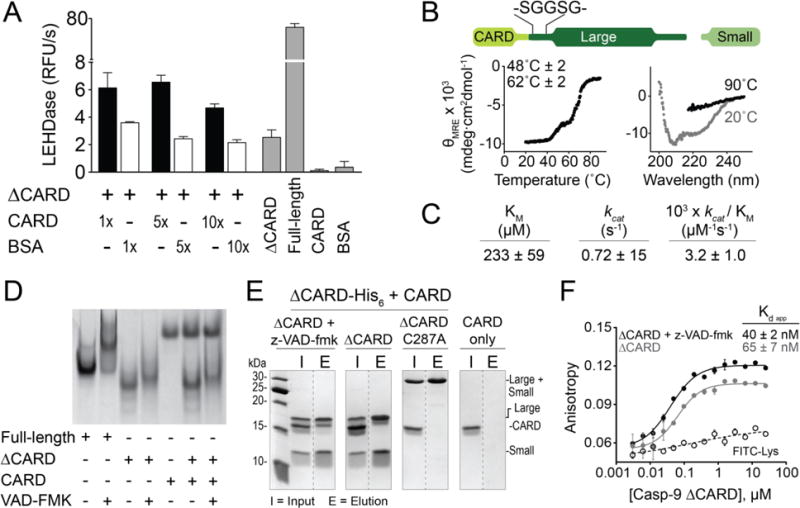
(A) In trans activity assay of ΔCARD caspase-9 with CARD. The presence of CARD enhances caspase-9 activity, but does not recapitulate the full activity of a full-length caspase-9. Error bars are SD from three trials done on three separate days.
(B) Thermal denaturation analysis (left) and circular dichroism spectra (right) of the Ser-Gly linker extension variant of caspase-9 showing the same melting transitions as that of full-length, cleaved caspase-9. Plots are representative of three trials. Tm values shown are means ± SEM of three trials done on three separated days.
(C) Caspase-9 Ser-Gly linker extension variant exhibits similar kinetic behaviors to wild-type full-length, cleaved caspase-9.
(D) Native gel electrophoresis showed no visible mobility shift, which indicate interaction between CARD and caspase-9 core (unbound/monomeric and z-VAD-fmk-bound/dimeric).
(E) His-tagged, dimeric, z-VAD-fmk-bound ΔCARD was able to pull down untagged CARD by Ni-NTA affinity assay, suggesting interaction between CARD and catalytic core of caspase-9.
(F) Fluorescence anisotropy shows binding of FITC-labeled CARD to both monomeric and dimeric (+ z-VAD-fmk) ΔCARD. Error bars are SD from three trials done on three separate days.
